Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 57(3); 2024 > Article
-
Original Article
Factors Associated With Long-term Retention in Antiretroviral Therapy Among People Living With HIV: Evidence From a Tertiary Hospital in Jakarta, Indonesia -
Ifael Yerosias Mauleti1
 , Krishna Adi Wibisana1
, Krishna Adi Wibisana1 , Djati Prasetio Syamsuridzal2
, Djati Prasetio Syamsuridzal2 , Sri Mulyati2
, Sri Mulyati2 , Vivi Lisdawati3
, Vivi Lisdawati3 , Ika Saptarini4,5
, Ika Saptarini4,5
 , Nurhayati4
, Nurhayati4 , Armedy Ronny Hasugian4
, Armedy Ronny Hasugian4 , Harimat Hendarwan4
, Harimat Hendarwan4
-
Journal of Preventive Medicine and Public Health 2024;57(3):252-259.
DOI: https://doi.org/10.3961/jpmph.23.512
Published online: April 30, 2024
- 1,083 Views
- 149 Download
1Department of Internal Medicine, Fatmawati General Hospital, Jakarta, Indonesia
2General Practitioner Staff, Fatmawati General Hospital, Jakarta, Indonesia
3Directorate of Human Resources, Education and Research, Fatmawati General Hospital, Jakarta, Indonesia
4Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency, Bogor, Indonesia
5Doctoral Program in Medical Sciences, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia
- Corresponding author: Ika Saptarini, Research Center for Preclinical and Clinical Medicine, National Research and Innovation Agency, Jalan Raya Jakarta-Bogor, Pakansari, Cibinong, Bogor 16915, Indonesia E-mail: ikas003@brin.go.id
Copyright © 2024 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives:
- This study investigated factors associated with the retention of people living with human immunodeficiency virus (HIV) on antiretroviral therapy (ART) during the first 3 years of treatment.
-
Methods:
- A retrospective study using electronic health records was conducted at a tertiary hospital in Jakarta, Indonesia. Adult HIV-positive patients who started ART from 2010 until 2020 were included. A binary logistic regression model was used to identify factors associated with ART retention in the first 3 years.
-
Results:
- In total, 535 respondents were included in the analysis. The ART retention rates for the first, second, and third years were 83.7%, 79.1%, and 77.2%, respectively. The multivariate analysis revealed a negative association between CD4 count when starting ART and retention. Patients with CD4 counts >200 cells/mL were 0.65 times less likely to have good retention than those with CD4 counts ≤200 cells/mL. The year of starting ART was also significantly associated with retention. Patients who started ART in 2010-2013 or 2014-2016 were less likely to have good retention than those who started ART in 2017-2020, with adjusted odds ratios of 0.52 and 0.40, respectively. Patients who received efavirenz-based therapy were 1.69 times more likely to have good retention than those who received nevirapine (95% confidence interval, 1.05 to 2.72).
-
Conclusions:
- Our study revealed a decline in ART retention in the third year. The CD4 count, year of enrollment, and an efavirenz-based regimen were significantly associated with retention. Patient engagement has long been a priority in HIV programs, with interventions being implemented to address this issue.
- Human immunodeficiency virus (HIV), which is arguably the most formidable communicable disease, represents a serious global public health issue. Infection weakens the host’s immune system, potentially leading to the development of acquired immune deficiency syndrome (AIDS) and high mortality rates if appropriate medical intervention is not provided. The World Health Organization (WHO) recommends antiretroviral therapy (ART) for all individuals who test positive for HIV. The provision of ART to HIV patients has led to increased survival rates, improved quality of life, and reduced HIV transmission. Every day, approximately 4000 people contract HIV, with 1100 of these cases occurring among young adults aged 15 years to 24 years. In 2021, it was reported that around 650 000 (with a range of 500 000 to 860 000) individuals died from AIDS-related causes, averaging about 1 person per minute [1]. The Asia-Pacific region saw a 9% reduction in new HIV infections from 2010 to 2018, with an estimated 310 000 to 340 000 new infections in 2018. In contrast, Indonesia experienced a marked increase in HIV cases from 510 000 to 640 000 in 2010 and 2018, respectively [2].
- Jakarta, the capital of Indonesia, has a population exceeding 10 million inhabitants. This region has the second-highest number of HIV/AIDS cases in the country, following East Java Province [3]. Fatmawati General Hospital, a tertiary hospital in Jakarta, houses the Wijaya Kusuma Clinic. This clinic, established in December 2006, specializes in the care of people living with HIV (PLHIV). At Fatmawati General Hospital, over 1100 PLHIV were registered as patients at the time of this study, and their data have been incorporated into electronic health records (EHRs). These records contain demographic information, social history, diagnoses, laboratory tests and results, and prescriptions. Such data can be utilized to predict disease risks, including those associated with HIV [4]. Additionally, EHRs hold the potential to forecast patient prognoses in HIV treatment, encompassing aspects such as retention in care.
- Retaining patients in medical care after initiating effective ART is crucial for maximizing therapeutic benefits and successfully treating HIV [5]. Poor retention is associated with adverse clinical outcomes, such as an unsuppressed viral load, a higher risk of transmission and mortality, and the potential development of drug resistance [6]. The Study on HIV Awal (Early) Test and Treat in Indonesia—known as HATI—reported a 6-month antiretroviral retention rate of 75%, relative to an ART initiation rate of 91% [7,8]. However, retention rates for ART at Fatmawati General Hospital are higher than the national average. The hospital has implemented a variety of strategies to improve HIV care, including HIV prevention programs and policies that support the use of telehealth and health informatics to improve patient follow-up. These strategies also encompass multi-month dispensing and the use of phone and text message reminders.
- Previous studies have established an inverse relationship between the duration of ART and patient retention in care: the longer the duration of ART, the lower the retention rate [9-11]. Therefore, it is essential to identify factors linked to poor retention to develop targeted interventions that can improve both individual and public health outcomes, as well as increase cost-effectiveness, particularly over the long term. Several patient-related factors have been recognized as predictors of poor retention in ART, including younger age, female sex, low socioeconomic status, lack of a regular healthcare provider, less advanced HIV disease, fewer comorbidities unrelated to HIV, and unmet psychosocial needs [12-15]. These factors may differ across countries. Our study was focused on investigating patient-related factors that influence retention in ART during the first 3 years of therapy.
INTRODUCTION
- Study Sample and Procedure
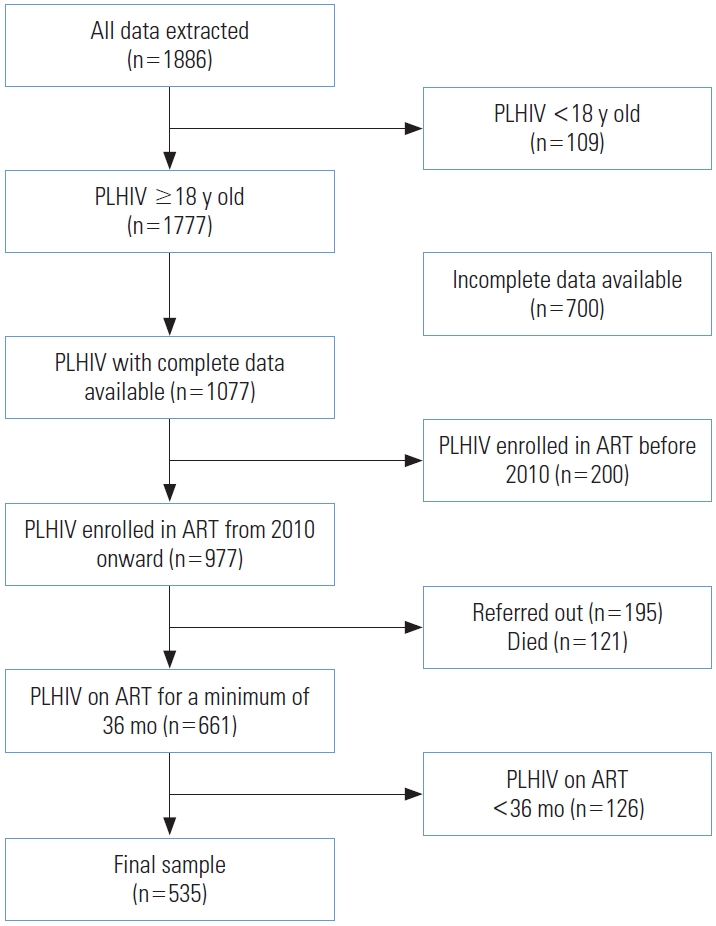
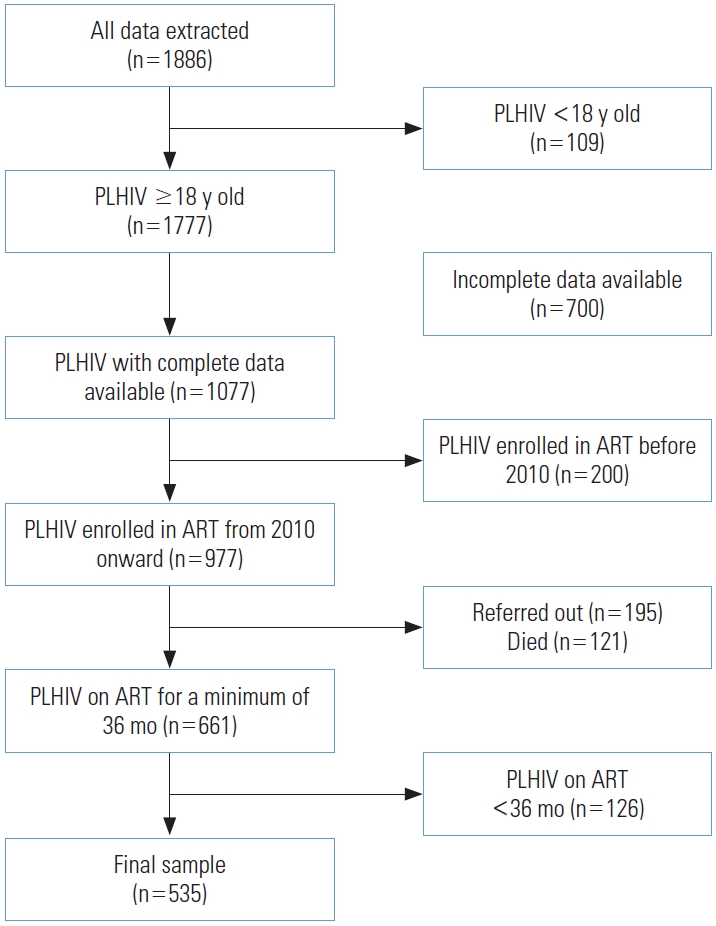
- The study was conducted at Fatmawati General Hospital in Jakarta, with data collected from EHR systems. These databases contain information on the treatment initiation and followup of HIV-positive patients receiving ART at the hospital. We extracted data from 1886 EHRs, of which 1777 records corresponded to patients aged 18 years or older. Complete data were available for 1077 of these patients. We excluded patients who initiated ART before 2010, those who had died, and those who were referred to other facilities. We further limited the study to patients who had been on ART for 36 months or more. Following data cleaning, a final sample of 535 respondents remained for analysis. The method of sample selection is depicted in Figure 1. Additionally, we compared the final sample used for analysis with the excluded patients, as detailed in Supplemental Material 1.
- A post-diagnosis session was held before each patient was enrolled in HIV care. Prior to starting ART, patients were screened for opportunistic infections. Furthermore, the initiation of ART was delayed in cases of active pulmonary tuberculosis. During the initial counseling session, the counselor collected information on the patient’s medical history, demographic characteristics, and any risk factors related to HIV.
- Measurements
- The dependent variable in this study was ART retention. Specifically, we assessed ART retention over the first 3 years. This was measured using the 3-month visit constancy approach, in which the year was divided into 4 quarters, each spanning 3 months. For each year, patients who attended at least 1 visit per quarter were considered to have good retention, whereas those who did not meet this threshold were deemed to have poor retention. We then determined retention across the full 3-year period. Patients who maintained good retention for all 3 years were classified as having good overall retention, while those who did not were classified as having poor overall retention.
- For the independent variables, we considered several factors from the demographic and clinical characteristics of patients at baseline, including age at ART enrollment, sex, year of ART initiation, WHO clinical stage, treatment regimen upon enrollment, CD4 count prior to starting ART, the use of tuberculosis post-exposure prophylaxis, and the presence of opportunistic infection. We categorized the age of participants into 2 groups: 18-45 years and over 45 years. Regarding the WHO clinical stage, patients were classified into 4 groups in accordance with available guidelines: stage 1, stage 2, stage 3, and stage 4. The treatment regimens at enrollment were grouped based on the type of non-nucleoside reverse-transcriptase inhibitors (NNRTIs) used: nevirapine-based and efavirenz-based. CD4 cell counts before the initiation of ART were divided into 2 categories: ≤200 cells/μL and >200 cells/μL. The year of ART initiation was coded into 3 periods: 2010-2013, 2014-2016, and 2017-2020.
- Statistical Analysis
- Descriptive statistics, including frequency distributions and percentages, were calculated for all independent and dependent variables. First, univariate analysis was employed to determine the characteristics of the study population. This was followed by a bivariate analysis to assess the association between each independent variable and ART retention. In the final model, a multivariate analysis was performed using log-binomial regression. We considered the inclusion of all independent variables—age, sex, CD4 count at ART initiation, WHO clinical stage, year of ART initiation, tuberculosis post-exposure prophylaxis, treatment regimen at enrollment, and the presence of opportunistic infections—based on their availability in the dataset and relevance as indicated by previous studies. Crude odds ratios, adjusted odds ratios (aORs), 95% confidence intervals (CIs), and p-values were calculated. For the multivariate analysis, a p-value of less than 0.05 was considered to indicate statistical significance. All analysis was conducted using Stata version 15.1 (StataCorp., College Station, TX, USA).
- Ethics Statement
- This study received ethical clearance from the Institutional Review Board of Fatmawati General Hospital (No. PP.08.02/D.XXI.18/75/2023). It relied exclusively on information from an EHR database and clinical register, without any direct patient interactions or specific inquiries for the study. Consequently, the data were strictly secondary in nature, rendering informed consent unnecessary. Patient identities were not accessed, ensuring that confidentiality was preserved.
METHODS
- After data cleaning, a total of 535 patients were included in the analysis. All baseline demographic and clinical characteristics were incorporated as independent variables. Table 1 summarizes the characteristics of adult patients who were HIV-positive and had initiated ART. Participants were primarily male (71.4%) and 18-44 years old (86.9%) at the time of ART enrollment. Two-thirds (67.1%) of the participants had CD4 levels of 200 cells/μL or lower prior to starting ART. More than half of the participants (58.7%) exhibited moderate symptoms of HIV and were classified as WHO stage 3 at the time of presentation. Less than 10% of the individuals were at stage 4 when they began ART. Over three-quarters of the participants (79.1%) were prescribed an efavirenz-based regimen as their initial ART, and approximately one-third (34.6%) were found to have opportunistic infections.
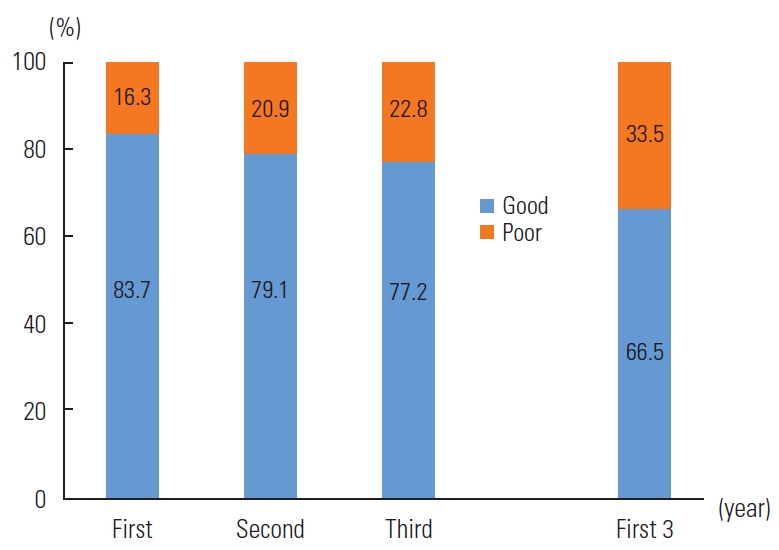
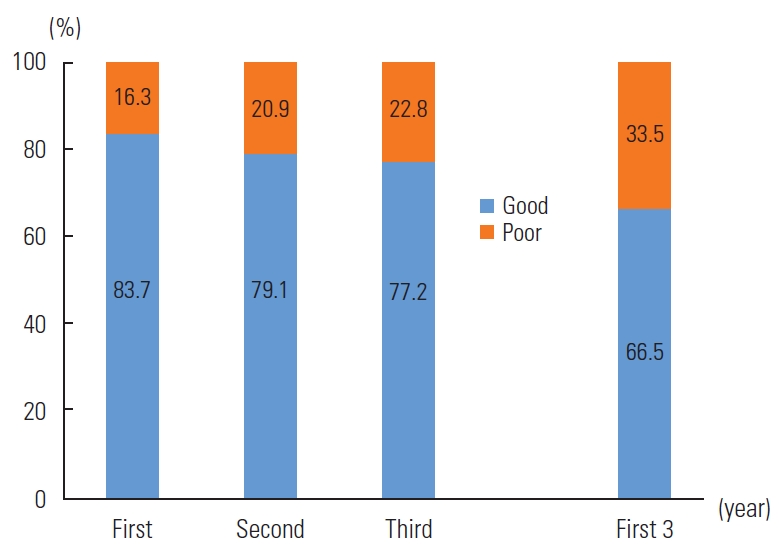
- Figure 2 illustrates the retention rates over the initial 3 years of ART. The retention rate in the first year stood at 83.7%, which declined to 79.1% in the second year and fell further to 77.2% in the third year. Overall, the proportion of good retention in the first 3 years was 66.6%.
- Table 2 presents the bivariate and multivariate analyses of factors associated with ART retention within the first 3 years. The bivariate analysis identified sex, CD4 count at ART initiation, year of ART initiation, and initial treatment regimen as significant predictors of ART retention during this period. Nevertheless, we opted to consider all variables for potential inclusion in the multivariate analysis. This analysis revealed that a higher CD4 count before starting ART was inversely correlated with retention in the first 3 years of treatment. Specifically, patients with a CD4 count greater than 200 cells/μL were 35% less likely to maintain good retention compared to those with a CD4 count of 200 cells/mL or less (aOR, 0.65; 95% CI, 0.43 to 0.98). Patients who began ART between 2010 and 2013 had a 48% lower likelihood of good retention compared to those who started between 2017 and 2020 (aOR, 0.52; 95% CI, 0.31 to 0.84). Similarly, patients who initiated ART between 2014 and 2016 were 60% less likely to have good retention than those who started between 2017 and 2020 (aOR, 0.40; 95% CI, 0.24 to 0.66). Regarding the initial regimen, our analysis indicated that patients receiving efavirenz-based treatment were 69% more likely to achieve good retention than those on a nevirapine-based regimen (aOR, 1.69; 95% CI, 1.05 to 2.72).
RESULTS
- This study examined the proportions of ART retention over the initial 3 years and the factors associated with them among PLHIV at Fatmawati General Hospital. We observed a decline in ART retention throughout the first 3 years of treatment. This suggests that a longer duration of ART is linked to a lower likelihood of retention. A systematic review conducted in subSaharan Africa, which assessed retention rates at 6 months, 12 months, and 24 months, reported similar results [16]. Likewise, a previous retrospective cohort study of adults (≥18 years) initiating ART in African countries from 2003 to 2010 found that retention proportions diminished from the first year to the fourth year of therapy [10]. Another retrospective cohort study in Nigeria, covering the years 2016 to 2019, demonstrated that retention rates decreased in the second and third years compared to the first year [11]. Several factors may contribute to this decline in ART retention. High geographic mobility among PLHIV, particularly within certain populations, may play a role [17]. The development of side effects and the demands of frequent medical appointments might also lead to lower retention rates [18]. Additionally, some patients may experience a temporary improvement in their clinical condition after starting treatment, which could lead them to prematurely discontinue ART. However, it is essential for individuals living with HIV to receive consistent and lifelong therapy. Despite efforts to improve retention in care over time, this study, along with prior research, indicates that the impact of these interventions on treatment adherence has been suboptimal. Thus, service delivery methods must be adapted to enable better outcomes, such as increased retention. A study from 2022 highlighted the use of effective service delivery models, including the identification of individual barriers to treatment continuity and the employment of a case management strategy to tackle these issues. Documented innovations and best practices support these methods [19].
- The present study indicated that CD4 count before the initiation of ART was negatively associated with ART retention in the first 3 years of treatment. Specifically, patients with a CD4 count greater than 200 cells/μL were less likely to exhibit good retention compared to those with a count of 200 cells/μL or less. This observation aligns with another study that reported a higher retention rate among patients with lower CD4 counts, which in turn contributes to more effective HIV management [6]. We hypothesize that individuals who start ART while already ill, as might be the case among PLHIV with lower CD4 counts, may value the treatment more highly than those who are free of infection. Accordingly, those with opportunistic infections may demonstrate greater adherence and engagement with their healthcare. Conversely, a previous study found that individuals with a CD4 count above 200 cells/μL at the initiation of treatment had better retention than those with a count below this threshold [20]. Our research also indicates that the year of ART enrollment was a factor in retention over the first 3 years. Patients who began ART in 2010-2013 or 2014-2016 were less likely to maintain good retention compared to those who started between 2017 and 2020. This trend likely reflects improvements in ART management over time. The number of PLHIV starting ART has meaningfully increased, leading to overcrowded healthcare facilities, longer wait times for appointments, and reduced time for counseling and clinical care for new patients. Nevertheless, substantial advancements have been made in global ART programs to improve the long-term retention of PLHIV in both community and hospital settings. These advancements encompass the introduction of diary cards, directly observed therapy, food ration provisions, engagement of treatment supporters, and the use of cell phone short message services [21]. Strategies such as HIV prevention programs and policies that promote telehealth and health informatics may also improve follow-up processes. These strategies include multi-month dispensing and call and text reminders, which have the potential to improve ART retention among patients with HIV.
- The present study revealed that patients receiving an efavirenz-based regimen were 1.69 times more likely to maintain good retention compared to those on a nevirapine-based regimen. Efavirenz, an NNRTI, functions by binding to a non-catalytic site of the HIV reverse transcriptase enzyme. This binding inhibits the enzyme’s DNA polymerase activity, preventing HIV replication [22]. Research conducted in Yaoundé, Cameroon, found that adherence to ART was higher with an efavirenz-based regimen than with a nevirapine-based approach. The greater adherence in patients on the efavirenz regimen may be attributed to the dosing frequency differences between the 2 regimens. Those on the efavirenz regimen benefit from a single, convenient daily dose, whereas those on the nevirapine regimen require doses twice daily. These results suggest that the efavirenz-based regimen is the preferable ART combination among NNRTIs [23].
- The primary strength of this study lies in its documentation of long-term ART retention at a tertiary hospital, as the findings may be relevant to other hospitals with similar characteristics. Additionally, the study leveraged data from EHRs, which are routinely employed to document medical treatment. This approach is both cost-effective and well-monitored. Nonetheless, it is crucial to consider certain limitations when interpreting the results. Our analysis of retention in care was limited by the narrow range of variables available in the data set. Consequently, we were unable to account for all possible confounders and predictors in our analysis. Furthermore, our study did not address factors such as socioeconomic status, access to health facilities, comorbidities, and lifestyle variables that may affect retention, since these were not captured in the data. The study also omitted clinician-related factors that could influence ART retention, again due to the constraints of data availability.
- In conclusion, our study corroborates the results of prior research, which show a decline in ART retention over the course of 3 years of treatment. We found that a higher CD4 count at the initiation of ART was inversely associated with retention. Additionally, patients on an efavirenz-based regimen demonstrated higher retention compared to those on a nevirapine-based regimen. Moreover, the year of ART initiation was positively associated with retention during the first 3 years of treatment. The insights gleaned from this study could be instrumental in the development of HIV treatment, care, and support programs that are both effective and efficient. Furthermore, the findings may prompt additional research on ART retention that incorporates sociodemographic barriers and clinician-related factors.
DISCUSSION
Supplemental Materials
Supplemental Material 1.
-
Conflict of Interest
The authors have no conflicts of interest associated with the material presented in this paper.
-
Funding
The study received funding from the Indonesia Endowment Fund for Education (LPDP).
-
Author Contributions
Conceptualization: Mauleti IY. Data curation: Mauleti IY, Wibisana KA, Syamsuridzal DP, Mulyati S. Formal analysis: Saptarini I. Funding acquisition: Lisdawati V, Saptarini I. Methodology: Mauleti IY, Saptarini I, Nurhayati, Hasugian AR, Hendarwan H. Project administration: Lisdawati V, Saptarini I. Visualization: Saptarini I, Nurhayati, Hasugian AR, Hendarwan H. Writing – original draft: Mauleti IY, Saptarini I. Writing – review & editing: Mauleti IY, Wibisana KA, Syamsuridzal DP, Mulyati S, Lisdawati V, Saptarini I, Nurhayati, Hasugian AR, Hendarwan H.
Notes
Acknowledgements
- 1. UNAIDS. UNAIDS global AIDS update 2022 [cited 2023 Oct 1]. Available from: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids-update
- 2. UNAIDS. The response to HIV in Asia and the Pacific — global AIDS update 2019; 2019 [cited 2023 Oct 1]. Available from: https://www.unaids.org/en/resources/documents/2019/2019-global-AIDS-update_asia-pacific
- 3. Ministry of Health of Indonesia. Infodatin HIV AIDS [cited 2023 Oct 1]. Available from: https://care.pkbi.or.id/download/file/infodatin-2020-HIV.pdf (Indonesian)
- 4. Chen M, Tan X, Padman R. Social determinants of health in electronic health records and their impact on analysis and risk prediction: a systematic review. J Am Med Inform Assoc 2020;27(11):1764-1773. https://doi.org/10.1093/jamia/ocaa143ArticlePubMedPMC
- 5. Adekanmbi O, Ilesanmi S, Ogunbosi B, Moradeyo D, Lakoh S. Retention in care among patients attending a large HIV clinic in Nigeria who were treated for tuberculosis. J Int Assoc Provid AIDS Care 2022;21: 23259582221124826. https://doi.org/10.1177/23259582221124826ArticlePubMedPMC
- 6. Shah GH, Etheredge GD, Nkuta LM, Waterfield KC, Ikhile O, Ditekemena J, et al. Factors associated with retention of HIV patients on antiretroviral therapy in care: evidence from outpatient clinics in two provinces of the democratic Republic of the Congo (DRC). Trop Med Infect Dis 2022;7(9):229. https://doi.org/10.3390/tropicalmed7090229ArticlePubMedPMC
- 7. Januraga PP, Reekie J, Mulyani T, Lestari BW, Iskandar S, Wisaksana R, et al. The cascade of HIV care among key populations in Indonesia: a prospective cohort study. Lancet HIV 2018;5(10):e560-e568. https://doi.org/10.1016/S2352-3018(18)30148-6ArticlePubMed
- 8. Subronto YW, Kusmayanti NA, Januraga PP, Dewa Wirawan LN, Wisaksana R, Sukmaningrum E, et al. Simplified clinical algorithm for immediate antiretroviral therapy initiation: the HATI [HIV awal (early) Test & Treat in Indonesia] implementation research in Indonesia. Indian J Med Res 2022;156(6):729-741. https://doi.org/10.4103/ijmr.ijmr_239_23ArticlePubMed
- 9. Long L, Kuchukhidze S, Pascoe S, Nichols BE, Fox MP, Cele R, et al. Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: a rapid systematic review. J Int AIDS Soc 2020;23(11):e25640. https://doi.org/10.1002/jia2.25640ArticlePubMedPMC
- 10. Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenga M, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Health 2014;19(12):1397-1410. https://doi.org/10.1111/tmi.12386ArticlePubMedPMC
- 11. Ibiloye O, Jwanle P, Masquillier C, Van Belle S, Jaachi E, Amoo O, et al. Long-term retention and predictors of attrition for key populations receiving antiretroviral treatment through community-based ART in Benue State Nigeria: a retrospective cohort study. PLoS One 2021;16(11):e0260557. https://doi.org/10.1371/journal.pone.0260557ArticlePubMedPMC
- 12. Yehia BR, Stewart L, Momplaisir F, Mody A, Holtzman CW, Jacobs LM, et al. Barriers and facilitators to patient retention in HIV care. BMC Infect Dis 2015;15: 246. https://doi.org/10.1186/s12879-015-0990-0ArticlePubMedPMC
- 13. Rooks-Peck CR, Adegbite AH, Wichser ME, Ramshaw R, Mullins MM, Higa D, et al. Mental health and retention in HIV care: a systematic review and meta-analysis. Health Psychol 2018;37(6):574-585. https://doi.org/10.1037/hea0000606ArticlePubMedPMC
- 14. Lee S, Lee SH, Lee SJ, Kim KH, Lee JE, Cho H, et al. Predictors of poor retention in care of HIV-infected patients receiving antiretroviral therapy in Korea: five-year hospital-based retrospective cohort study. J Korean Med Sci 2016;31(3):376-381. https://doi.org/10.3346/jkms.2016.31.3.376ArticlePubMedPMC
- 15. Holtzman CW, Shea JA, Glanz K, Jacobs LM, Gross R, Hines J, et al. Mapping patient-identified barriers and facilitators to retention in HIV care and antiretroviral therapy adherence to Andersen’s Behavioral Model. AIDS Care 2015;27(7):817-828. https://doi.org/10.1080/09540121.2015.1009362ArticlePubMedPMC
- 16. Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 2007;4(10):e298. https://doi.org/10.1371/journal.pmed.0040298ArticlePubMedPMC
- 17. Vuylsteke B, Semdé G, Auld AF, Sabatier J, Kouakou J, EttiègneTraoré V, et al. Retention and risk factors for loss to follow-up of female and male sex workers on antiretroviral treatment in Ivory Coast: a retrospective cohort analysis. J Acquir Immune Defic Syndr 2015;68(Suppl 2):S99-S106. https://doi.org/10.1097/QAI.0000000000000442ArticlePubMedPMC
- 18. Tegegne AS, Ndlovu P, Zewotir T. Factors affecting first month adherence due to antiretroviral therapy among HIV-positive adults at Felege Hiwot Teaching and Specialized Hospital, north-western Ethiopia; a prospective study. BMC Infect Dis 2018;18(1):83. https://doi.org/10.1186/s12879-018-2977-0ArticlePubMedPMC
- 19. Atuhaire L, Shumba CS, Mapahla L, Nyasulu PS. A retrospective cross sectional study assessing factors associated with retention and non-viral suppression among HIV positive FSWs receiving antiretroviral therapy from primary health care facilities in Kampala, Uganda. BMC Infect Dis 2022;22(1):642. https://doi.org/10.1186/s12879-022-07614-wArticlePubMedPMC
- 20. Ajeh RA, Gregory HE, Thomas EO, Noela NA, Dzudie A, Jules AN, et al. Determinants of retention in HIV antiretroviral treatment (ART) in the Cameroon International epidemiology Database to Evaluate AIDS (IeDEA) study clinics: the context of the HIV treat all strategy in Cameroon. Pan Afr Med J 2021;40: 129. https://doi.org/10.11604/pamj.2021.40.129.22642ArticlePubMedPMC
- 21. Damulak PP, Ismail S, Abdul Manaf R, Mohd Said S, Agbaji O. Interventions to improve adherence to antiretroviral therapy (ART) in sub-Saharan Africa: an updated systematic review. Int J Environ Res Public Health 2021;18(5):2477. https://doi.org/10.3390/ijerph18052477ArticlePubMedPMC
- 22. McDonagh EM, Lau JL, Alvarellos ML, Altman RB, Klein TE. PharmGKB summary: efavirenz pathway, pharmacokinetics. Pharmacogenet Genomics 2015;25(7):363-376. https://doi.org/10.1097/FPC.0000000000000145ArticlePubMedPMC
- 23. Chendi BH, Okomo Assoumou MC, Jacobs GB, Yekwa EL, Lyonga E, Mesembe M, et al. Rate of viral load change and adherence of HIV adult patients treated with Efavirenz or Nevirapine antiretroviral regimens at 24 and 48weeks in Yaoundé, Cameroon: a longitudinal cohort study. BMC Infect Dis 2019;19(1):194. https://doi.org/10.1186/s12879-019-3824-7ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite