ABSTRACT
-
Objectives
- The aim of this study was to present the baseline results of a pilot project conducted to evaluate the effectiveness of lung cancer screening using low-dose chest computed tomography (CT) in regions with excessive radon levels in the Republic of Kazakhstan.
-
Methods
- In total, 3671 participants were screened by low-dose chest CT. Current, former, and never-smokers who resided in regions with elevated levels of radon in drinking water sources and indoor air, aged between 40 and 75 with no history of any cancer, and weighing less than 140 kg were included in the study. All lung nodules were categorized according to the American College of Radiology Lung Imaging Reporting and Data System (Lung-RADS 1.0).
-
Results
- Overall, 614 (16.7%) participants had positive baseline CT findings (Lung-RADS categories 3 and 4). Seventy-four cancers were detected, yielding an overall cancer detection rate of 2.0%, with 10.8% (8/74) stage I and a predominance of stage III (59.4%; 44/74). Women never-smokers and men current smokers had the highest cancer detection rates, at 2.9% (12/412) and 6.1% (12/196), respectively. Compared to never-smokers, higher odds ratios (ORs) of lung cancer detection were found in smokers (OR,2.48; 95% confidence interval [CI], 1.52 to 4.05, p<0.001) and former smokers (OR, 2.32; 95% CI, 1.06 to 5.06, p=0.003). The most common histologic type of cancer was adenocarcinoma (58.1%).
-
Conclusions
- Implementation of low-dose CT screening for lung cancer in regions with elevated radon levels is an effective method for both smokers and never-smokers.
-
Keywords: Lung neoplasms, Radon, Mass screening, Kazakhstan
INTRODUCTION
- Lung cancer is the leading cause of cancer-related mortality in the world [1]. The International Agency for Research on Cancer ranks Kazakhstan as having one of the highest rates of lung cancer incidence in the world among the Asian countries closest to Eastern Europe [2].
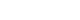
- However, lung cancer incidence and mortality rates are heterogeneous in the territory of Kazakhstan, exceeding the national level (20.1 and 12.3/100 000, respectively) in 7 of 17 regions: Akmola (33.8 and 25.0/100 000), East Kazakhstan (32.1 and 23.9/100 000), Pavlodar (36.4 and 23.3/100 000), North Kazakhstan (44.6 and 18.2/100 000), West Kazakhstan (27.4 and 17.8/100 000), Karaganda (27.4 and 14.2/100 000), and Kostanay (25.9 and 12.1/100 000) [3]. According to the report of the International Atomic Energy Agency, the 7 above-mentioned regions have increased levels of radon in drinking water sources and indoor air (Figure 1) [4].
- As known, cigarette smoking is the main risk factor for lung cancer, while radon is responsible for lung cancer in never-smokers [5–7]. According to the World Nuclear Association, the Republic of Kazakhstan is a leader in the world’s reserves of uranium ores, with 12% of the world’s uranium resources and an expanding mining sector, producing about 22 808 tons in 2019 [8].
- In 2019, 3743 new cases of lung cancer were reported in Kazakhstan, more than half of which (n=1989, 53.1%) were registered in the above-mentioned regions. Stage I was diagnosed only in 6.9% of patients, while the largest proportion of patients (45.8%) had stage III disease [3].
- The aim of our study was to conduct lung cancer screening with low-dose computed tomography (CT) among individuals living in unfavorable regions in terms of radon exposure.
METHODS
- We performed low-dose CT screening for lung cancer during a 2-year period, from June 1, 2018 to May 31, 2020 as a part of a pilot project. There was 1 round in 2 stages of 1 year each, in different regions of Kazakhstan. Our prospective multicenter study included participants living in regions with rates of mortality and incidence from lung cancer that exceed the nationwide level and high rates of radon pollution in drinking water sources (more than 60 Bq/L) and indoor air (more than 200 Bq/m3). On average, the excess of the norms in the selected regions was 7.8% for water sources and 36.8% for indoor air, with maximum values of 11.5% for water sources and 47.6% for indoor air.
- The population size in our study was 1 200 000 individuals; given a 95% confidence interval (CI) and a power of 80%, a sample size of 5000 participants was considered representative.
- The recruiting process was conducted by primary care organizations, which invited 5000 eligible participants via phone calls. Of the eligible participants, 943 (18.9%) refused to participate and 4057 (81.1%) agreed. Of those who agreed, 3675 (90.6%) individuals attended the clinic for the study, and 382 (9.4%) changed their minds and refused. Another 4 participants did not sign informed consent and did not complete the questionnaire. As a result, 3671 participants completed the questionnaire that included personal data, information about permanent residence, smoking history, and heredity and completed the low-dose CT examination. The participation rate was 73.4% The study involved adults aged 40–75, with no history of any cancer or severe comorbid conditions, no history of a CT examination during the year, and a weight of less than 140 kg. Smoking status was not applied as a criterion for inclusion.
- Scanning was performed at 3 oncological centers using various CT scanners (Revolution EVO; GE Healthcare, Chicago, IL, USA; Optima GE Healthcare, Chicago, IL, USA, and Aquilion Prime, Toshiba, Tokyo, Japan) with different numbers of detectors (16–128) and slice thickness not exceeding 1.25 mm. All scanners had a low-dose scanning protocol, with the machine set at 120 kV and 10–40 mA. The effective radiation dose did not exceed 1 mSv (according to the order for preventive examinations of the population in Kazakhstan), and the average effective radiation dose was 0.69±0.21 mSv. CT scanning was performed during the inspiratory phase, from the apex to the base of the lungs; no contrast agent was injected.
- All low-dose CT images were sent to the Republican Research Institute of Oncology and Radiology using a picture archiving and communication system. The interpretation was done by 2 independent thoracic radiologists, with 6 years and 35 years of experience, respectively. In case of a discrepancy in the findings, the third reader gave his opinion and was the final arbiter. Lung nodules were classified according to Lung Imaging Reporting and Data System (Lung-RADS 1.0).
- Categories 3 and 4 were designated as positive (at least 1 non-calcified nodule 6 mm or larger or a pure ground-glass nodule 20 mm or larger).
- Statistical Analysis
- Data were analyzed using SPSS version 27 (IBM Corp., Armonk, NY, USA). The sample size was calculated using the EpiInfo calculator. Odds ratios (ORs) were used for risk estimation of lung cancer detection according to smoking history. We calculated the ORs of lung cancer in 3 pairs of groups: current smokers versus never-smokers, current smokers versus former smokers, and ex-smokers versus never-smokers. The chi-square test was applied to assess statistical differences between categorical variables. A p-value<0.05 was considered to indicate statistical significance.
- Ethics Statement
- The present study protocol was reviewed and approved by the Ethics Committee of the Kazakh Research Institute of Oncology and Radiology (approval No. 12/18). All participants were consulted about the procedure and signed informed consent.
RESULTS
- In total, 3671 participants were screened by unenhanced low-dose chest CT (Table 1).
- The distribution of detected nodules and Lung-RADS 1.0 categories at baseline according to smoking history are shown in Table 2. Overall, 614 (16.7%) participants had baseline positive CT findings. Among them, 525 (85.5%) individuals required follow-up, the timing of which ranged from 3 months to 6 months after the initial scan. Only 320 (60.9%) individuals underwent rescanning according to the designated period. Most pulmonary nodules did not increase in size and remained stable during the follow-up period, while 13 nodules disappeared. Eighty-nine (14.5%) patients needed additional examinations, followed by biopsy and/or surgery. Considering the peripheral location and appropriate access, 7 (7.8%) persons underwent transthoracic biopsy, in 1 case adenocarcinoma was confirmed. Considering the central location at the lung root and the connection with the large bronchus, 9 (10.1%) individuals underwent bronchoscopy, and cancer was confirmed in 100% of these cases. Seventy-three (82.1%) participants underwent surgery (wedge resection/lobectomy). The overall lung cancer detection rate in our study was 2.01%. More results about follow-up and outcomes are presented in Figure 2.
- The age range of participants diagnosed with lung cancer was 42–75 years (median, 60.4±8.7). Among the 74 cases of identified lung cancers, 34 (45.9%) were in smokers with an average smoking history of 56.9 pack-years; 8 (10.8%) were in former smokers with an average smoking status of 22.5 pack-years, and 32 (43.3%) were in never-smokers (Table 3). Compared to never-smokers, higher ORs for lung cancer detection were found in smokers (OR, 2.48; 95% CI, 1.52 to 4.05; p<0.001) and in former smokers (OR, 2.32; 95% CI, 1.06 to 5.06; p=0.003), while no statistically significant difference was found between never-smokers and former smokers (OR, 0.93; 95% CI, 0.43 to 2.04; p=0.865). However, slightly more than half of the participants with detected lung cancer were never-smokers and former smokers (54.1%) and they might have been missed if smoking status had been an obligatory inclusion criterion.
- The highest detection rate of lung cancer was in the 70–75 age group (4.6%; 19/412). The cancer detection rate among men was higher than that among women (3.1 and 2.1%, respectively). In the men population, the highest cancer detection rate was 6.1% in current smokers aged 60–69 years (12/196). Among the women population, the highest cancer detection rate was 2.9% in never-smokers 50–59 years (12/412).
- In 62 (83.8%) patients, the structure of the nodule was solid, ranging in size from 11 mm to 82 mm, with the average size of the nodule being 54.2 mm. In 12 (16.2%) patients, the nodules had a sub-solid structure, ranging in size from 11 mm to 45 mm, with an average nodule size of 28.5 mm.
- According to the pathology reports, 43 (58.1%) patients had adenocarcinoma, 28 (37.8%) participants had squamous cell carcinoma, and 3 (4.1%) patients had small cell lung cancer.
- The majority of patients (44; 59.4%) were diagnosed with stage III (IIIA: 32 [43.2%], IIIB: 8 [10.8%], and IIIC: 4 [5.4%]), while 21 (28.4%) patients had stage II (IIA: 9 [12.2%] and IIB: 12 [16.2%]). Only 8 (10,8%) patients had stage I (IA: 5 [6.8%] and IB: 3 [4%]). Stage IVA cancer was diagnosed in 1 (1.4%) patient.
- Besides lung nodules, other clinically significant findings were detected in 48% of patients (n=1762), including emphysema, old tuberculosis and a few cases of active pulmonary tuberculosis, bronchiectasis, pleural effusion, lymphadenopathy, coronary artery sclerosis and aortosclerosis, retrosternal goiter, and breast cancer.
DISCUSSION
- In our multicenter study, we present the baseline screening results of lung cancer in radon-contaminated regions of Kazakhstan. Despite the fact that smoking was not a mandatory criterion for inclusion in our study, we obtained a fairly high cancer detection rate of 2.0% at baseline, which is higher than in most lung cancer screening programs in Asia. For instance, in the Korean Lung Cancer Screening Project, the baseline lung cancer detection rate was 0.4%, and. The Taiwan, Shanghai, and Tokyo lung cancer screening programs showed rates of 1.41%, 1.23%, and 1.0%, respectively [9–12]. However, in the National Lung Screening Trial (NLST) and the Dutch-Belgian Lung Cancer Screening Trial, the overall rates of lung cancer detection were higher (2.4 and 3.2%, respectively) [13,14], which is most probably due to more rounds and the use of different inclusion criteria and nodule management protocols.
- Age and smoking history are the main selection criteria for screening, with different thresholds in various programs. We chose an age interval of 40–75 years based on the National Cancer Registry of Kazakhstan, according to which the peak incidence of lung cancer occurs in the age range of 55–70 years. Considering that in our study the majority of patients with lung cancer were diagnosed at stage III, as well as the possible slow growth of adenocarcinoma (which was also predominant), the lower age limit was 40 years.
- Smoking is undoubtedly the most important factor for lung cancer development [15,16]. According to Jha [17], roughly 450 million people will die from smoking between 2000 and 2050. Although large randomized trials have demonstrated the effectiveness of screening in high-risk populations including heavy smokers, these trials generally did not include former-smokers and never-smokers. Thus, the benefits of lung cancer screening for former-smokers and never-smokers are uncertain. More than 50% of lung cancers can be missed by following current lung cancer screening guidelines and inclusion criteria that include heavy smokers, especially in East Asia, where most of the missed lung cancer cases are among never-smokers [18,19]. Furthermore, several studies, mainly in Asian countries, have shown that among never-smokers (especially women), lung cancer is quite common and that low-dose CT screening helped to detect a significant number of lung cancers in this group [12,20–23]. In our study, the majority of men with lung cancer were smokers, while never-smokers predominated among women. Nonetheless, there is evidence that low-dose CT screening in never-smokers or light smokers was also effective and led to a decrease in mortality by up to 51% [20].
- In our study, 43.2% of the participants were never-smokers, who showed a 1.4% cancer detection rate; this rate is quite high, but understandable, since the study was conducted in a radon-polluted region. In a multicenter study in northwestern Spain that examined 69 never-smoking patients with lung cancer and evaluated the prevalence of adenocarcinoma, the researchers demonstrated a relationship between high levels of radon (237 Bq/m3) and the development of lung cancer [24]. According to a study by Peterson et al. [25], 13.6% of lung cancer deaths in Ontario, Canada are attributable to radon, and approximately 84% of those deaths occur in never-smokers. If indoor air radon concentrations above 200 Bq/m3 could be reduced to background levels, it is estimated that 91 lung cancer deaths could be prevented each year in Ontario.
- The definition of a positive result is also an important issue. We defined at least 1 non-calcified solid or partly solid pulmonary nodule ≥6 mm or pure ground-glass nodule ≥20 mm as a positive result, while most studies used the NLST criteria (any non-calcified nodules equal to or larger than 4 mm in any diameter). However, some researchers have concluded that the application of the NLST criteria is not entirely appropriate for Asian regions. Scientists from Korea came to the conclusion that the use of Lung-RADS in areas endemic for tuberculosis (such as Kazakhstan) leads to a decrease in specificity, due to the patterns of old tuberculosis, suggesting that some modification of Lung-RADS might be required for these regions [26].
- One of the main limitations of our study is that only 1 round was conducted. In the future, we plan to increase the number of rounds and possibly modify the criteria for evaluating a positive result, which will increase the percentage of cancers detected at early stages enabling timely and adequate treatment to reduce mortality. Unfortunately, according to the first-round results of our study, only 10.8% of cases were stage I, while most similar studies showed percentages of stage I at baseline ranging from 27.3% to 100% [27].
- Studies across the globe are still ongoing to evaluate the effectiveness and possible implementation of low-dose CT screening for lung cancer in national screening programs, as well as to determine the specific contingent that should be included in the study [28]. We believe that our research will not only improve the situation regarding lung cancer in Kazakhstan, but will also contribute to global data on the current problem.
- Our study showed that low dose CT screening is an effective method for actively detecting lung cancer, and there is an urgent need for its implementation.
ACKNOWLEDGEMENTS
This study was carried out as part of a pilot project to implement a complex plan to control cancer in the Republic of Kazakhstan.
We express our gratitude to the company Ekoservice-C, especially to the employee and expert of the International Nuclear Energy Agency Vladislav Bensman for kindly providing the report “Conducting radiation monitoring of rural settlements in 2008–2011 in the Republic of Kazakhstan.”
Notes
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Panina A, Kaidarova D, Amankulov J. Data curation: Zholdybay Z, Ainakulova A. Formal analysis: Panina A, Toleshbayev D, Zhakenova Z. Funding acquisition: None. Methodology: Zhakenova Z, Khozhayev A. Project administration: Amankulov J, Toleshbayev D. Visualization: Amankulov J, Toleshbayev D. Writing – original draft: Panina A, Ainakulova A. Writing – review & editing: Kaidarova D, Zholdybay Z, Amankulov J, Toleshbayev D, Zhakenova Z, Khozhayev A.
Figure 1The level of radon in drinking water sources and indoor air by regions of the Republic of Kazakhstan.
Figure 2Follow-up and outcome of baseline low-dose computed tomography imaging data. Lung-RADS, Lung Imaging Reporting and Data System; TTB, transthoracic biopsy.

Table 1Characteristics of the study participants1
|
Characteristics |
|
n (%) |
|
Gender |
Men |
1582 (43.1) |
|
Women |
2089 (56.9) |
|
|
Age (y) |
40–49 |
811 (22.1) |
|
50–59 |
1356 (37.0) |
|
60–69 |
1092 (29.7) |
|
70–75 |
412 (11.2) |
|
|
Smoking status |
Never-smokers |
2198 (59.9) |
|
Former smokers |
514 (14.2) |
|
Current smokers |
959 (26.1) |
|
|
Family history of lung cancer |
Present |
117 (3.2) |
|
Absent |
3554 (96.8) |
Table 2Lung-RADS (LR) 1.0 categories at baseline according to smoking history
|
Smoking history |
LR 1 |
LR 2 |
LR 3 |
LR 4A |
LR 4B |
Total |
|
Never smokers |
1536 (69.9) |
328 (14.9) |
275 (12.5) |
26 (1.2) |
33 (1.5) |
2198 |
|
Former smokers |
350 (68.1) |
73 (14.2) |
66 (12.8) |
14 (2.7) |
11 (2.1) |
514 |
|
Current smokers |
632 (65.9) |
138 (14.4) |
106 (11.1) |
44 (4.6) |
39 (4.1) |
959 |
|
Total |
2518 (68.6) |
539 (14.7) |
447 (12.2) |
84 (2.3) |
83 (2.2) |
3671 |
Table 3Characteristics of study participants with low-dose computed tomography-detected lung cancer
|
Variables |
|
n (%) |
|
Gender |
Men |
50 (67.6) |
|
Women |
24 (32.4) |
|
|
Age (y) |
40–49 |
9 (12.1) |
|
50–59 |
29 (39.2) |
|
60–69 |
17 (23.0) |
|
70–75 |
19 (25.7) |
|
|
Smoking status |
Never-smokers |
32 (43.3) |
|
Former smokers |
8 (10.8) |
|
Current smokers |
34 (45.9) |
|
|
Family history of lung cancer |
Present |
8 (10.8) |
|
Absent |
66 (89.2) |
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71(3):209-249ArticlePubMedPDF
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-E386ArticlePubMed
- 3. Kaidarova DR, Shatkovskaya OV, Zholdybay Z, Panina AS. Lung cancer epidemiology in the Republic of Kazakhstan. Oncol Radiol Kazakhstan 2019;2(52):10-16
- 4. Ministry of Environmental Protection of the Republic of Kazakhstan. Conducting radiation monitoring of rural settlements in 2008–2011. Almaty: Ministry of Environmental Protection of the Republic of Kazakhstan; 2011. p. 14-16 (Russian)
- 5. Pawel DJ, Puskin JS. The U.S. Environmental Protection Agency’s assessment of risks from indoor radon. Health Phys 2004;87(1):68-74ArticlePubMed
- 6. World Health Organization. WHO handbook on indoor radon: a public health perspective; 2009 [cited 2021 Nov 1]. Available from: https://www.who.int/publications/i/item/9789241547673
- 7. Corrales L, Rosell R, Cardona AF, Martín C, Zatarain-Barrón ZL, Arrieta O. Lung cancer in never smokers: the role of different risk factors other than tobacco smoking. Crit Rev Oncol Hematol 2020;148: 102895ArticlePubMed
- 8. World Nuclear Association. Uranium and nuclear power in Kazakhstan; 2021 [cited 2021 Nov 1]. Available from: https://world-nuclear.org/information-library/country-profiles/countries-g-n/kazakhstan.aspx
- 9. Lee JW, Kim HY, Goo JM, Kim EY, Lee SJ, Kim TJ, et al. Radiological report of pilot study for the Korean Lung Cancer Screening (K-LUCAS) project: feasibility of implementing lung imaging reporting and data system. Korean J Radiol 2018;19(4):803-808ArticlePubMedPMCPDF
- 10. Wu FZ, Huang YL, Wu CC, Tang EK, Chen CS, Mar GY, et al. Assessment of selection criteria for low-dose lung screening CT among Asian ethnic groups in Taiwan: from mass screening to specific risk-based screening for non-smoker lung cancer. Clin Lung Cancer 2016;17(5):e45-e56ArticlePubMed
- 11. Fan L, Wang Y, Zhou Y, Li Q, Yang W, Wang S, et al. Lung cancer screening with low-dose CT: baseline screening results in Shanghai. Acad Radiol 2019;26(10):1283-1291ArticlePubMed
- 12. Kakinuma R, Muramatsu Y, Asamura H, Watanabe SI, Kusumoto M, Tsuchida T, et al. Low-dose CT lung cancer screening in never-smokers and smokers: results of an eight-year observational study. Transl Lung Cancer Res 2020;9(1):10-22ArticlePubMedPMC
- 13. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395-409ArticlePubMedPMC
- 14. Yousaf-Khan U, van der Aalst C, de Jong PA, Heuvelmans M, Scholten E, Lammers JW, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5-year screening interval. Thorax 2017;72(1):48-56ArticlePubMed
- 15. de Groot P, Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am 2012;50(5):863-876ArticlePubMed
- 16. Samet JM. Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin 2013;23(2):103-112PubMed
- 17. Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009;9(9):655-664ArticlePubMedPDF
- 18. Kerpel-Fronius A, Tammemägi M, Cavic M, Henschke C, Jiang L, Kazerooni E, et al. Screening for lung cancer in individuals who never smoked: an international association for the study of lung cancer early detection and screening committee report. J Thorac Oncol 2022;17(1):56-66ArticlePubMed
- 19. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019;85(1):8ArticlePubMedPMC
- 20. Nawa T. Low-dose CT screening for lung cancer reduced lung cancer mortality in Hitachi City. Int J Radiat Biol 2019;95(10):1441-1446ArticlePubMed
- 21. Chen CY, Chen CH, Shen TC, Cheng WC, Hsu CN, Liao CH, et al. Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J Formos Med Assoc 2016;115(3):163-170ArticlePubMed
- 22. Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol 2007;25(5):472-478ArticlePubMedPMC
- 23. Kang HR, Cho JY, Lee SH, Lee YJ, Park JS, Cho YJ, et al. Role of low-dose computerized tomography in lung cancer screening among never-smokers. J Thorac Oncol 2019;14(3):436-444ArticlePubMed
- 24. Ruano-Ravina A, Prini-Guadalupe L, Barros-Dios JM, Abal-Arca J, Leiro-Fernández V, González-Silva AI, et al. Exposure to residential radon and lung cancer in never-smokers: the preliminary results of the LCRINS study. Arch Bronconeumol 2012;48(11):405-409ArticlePubMed
- 25. Peterson E, Aker A, Kim J, Li Y, Brand K, Copes R. Lung cancer risk from radon in Ontario, Canada: how many lung cancers can we prevent? Cancer Causes Control 2013;24(11):2013-2020ArticlePubMedPMCPDF
- 26. Kim H, Kim HY, Goo JM, Kim Y. Lung cancer CT screening and lung-RADS in a tuberculosis-endemic country: the Korean Lung Cancer Screening Project (K-LUCAS). Radiology 2020;296(1):181-188ArticlePubMed
- 27. Triphuridet N, Henschke C. Landscape on CT screening for lung cancer in Asia. Lung Cancer (Auckl) 2019;10: 107-124PubMedPMC
- 28. Saul EE, Guerra RB, Saul ME, da Silva LL, Aleixo GF, Matuda RM, et al. The challenges of implementing low-dose computed tomography for lung cancer screening in low-and middle-income countries. Nat Cancer 2020;1(12):1140-1152ArticlePubMedPDF
Citations
Citations to this article as recorded by

- Lung cancer screening for never smokers: current evidence and future directions
Kay Choong See
Singapore Medical Journal.2024;[Epub] CrossRef - Secondary prevention and treatment innovation of early stage non-small cell lung cancer: Impact on diagnostic-therapeutic pathway from a multidisciplinary perspective
Giulia Pasello, Daniela Scattolin, Laura Bonanno, Francesca Caumo, Andrea Dell'Amore, Elena Scagliori, Mariaenrica Tinè, Fiorella Calabrese, Gaetano Benati, Matteo Sepulcri, Cristina Baiocchi, Michele Milella, Federico Rea, Valentina Guarneri
Cancer Treatment Reviews.2023; 116: 102544. CrossRef - Performance of Lung-RADS in different target populations: a systematic review and meta-analysis
Yifei Mao, Jiali Cai, Marjolein A. Heuvelmans, Rozemarijn Vliegenthart, Harry J. M. Groen, Matthijs Oudkerk, Marleen Vonder, Monique D. Dorrius, Geertruida H. de Bock
European Radiology.2023; 34(3): 1877. CrossRef
 , Dilyara Kaidarova1,2
, Dilyara Kaidarova1,2 , Zhamilya Zholdybay2
, Zhamilya Zholdybay2 , Akmaral Ainakulova1,2
, Akmaral Ainakulova1,2 , Jandos Amankulov1,2
, Jandos Amankulov1,2 , Dias Toleshbayev1,2
, Dias Toleshbayev1,2 , Zhanar Zhakenova2
, Zhanar Zhakenova2 , Arman Khozhayev2
, Arman Khozhayev2


 KSPM
KSPM



 PubReader
PubReader ePub Link
ePub Link Cite
Cite