Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 55(2); 2022 > Article
-
Original Article
Determinants of Optimal Breastfeeding Practices in Indonesia: Findings From the 2017 Indonesia Demographic Health Survey -
Siti Nurokhmah
 , Setyaningrum Rahmawaty
, Setyaningrum Rahmawaty , Dyah Intan Puspitasari
, Dyah Intan Puspitasari
-
Journal of Preventive Medicine and Public Health 2022;55(2):182-192.
DOI: https://doi.org/10.3961/jpmph.21.448
Published online: February 23, 2022
Department of Nutrition Science, Faculty of Health Science, Universitas Muhammadiyah Surakarta, Surakarta, Indonesia
- Corresponding author: Siti Nurokhmah, Department of Nutrition Science, Faculty of Health Science, Universitas Muhammadiyah Surakarta, Kampus 1 - Pabelan, Surakarta 57102, Indonesia, E-mail: siti.nurokhmah@ums.ac.id
Copyright © 2022 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Understanding the factors influencing mothers’ decision to breastfeed their infants is essential to formulate effective breastfeeding interventions. This study explored the determinants of optimal breastfeeding indicators in Indonesia.
-
Methods
- We used the 2017 Indonesia Demographic and Health Survey to analyze factors associated with early initiation of breastfeeding, exclusive breastfeeding (EBF), and continued breastfeeding at 1 year (CBF-1) and 2 years (CBF-2). Multivariate logistic regression models were used to examine bio-demographic, socio-cultural, and behavioral characteristics associated with breastfeeding after considering the survey design effect.
-
Results
- The risk of delayed breastfeeding initiation was higher among infants who were born smaller, first-born children, were delivered via cesarean delivery, and did not have immediate skin-to-skin contact (p<0.01). Infant’s age, birth pattern, household wealth index, and the mother’s occupation and smoking status were predictors of EBF (p<0.05). CBF-1 was less common among first-time mothers and those working in the non-agricultural sector, mothers from wealthier families, and mothers who had cesarean deliveries (p<0.01). Infant’s age was negatively associated with CBF-2 (adjusted odds ratio [aOR], 0.85; 95% confidence interval [CI], 0.74 to 0.99). Mothers attending college were less likely to practice CBF-2 than those with no education or primary education (aOR, 0.45; 95% CI, 0.26 to 0.77). The absence of postnatal visits was a risk factor for CBF-1 and CBF-2 (p<0.05).
-
Conclusions
- Breastfeeding interventions in Indonesia should pay particular attention to at-risk groups such as women from wealthier families, working outside the agricultural sector, and with a higher education level. Nutrition-sensitive programs (e.g., postnatal care and smoking cessation) should also be encouraged.
- Although the significant advantages of breastfeeding for children’s survival, growth, and development have been widely recognized, many mothers in developing countries do not breastfeed their infants as recommended by the World Health Organization (WHO) and the United Nations Children’s Fund [1]. The recommendation for optimal breastfeeding includes early initiation of breastfeeding (EIBF), exclusive breastfeeding (EBF), and continued breastfeeding to age 2 years or beyond [2]. Optimal breastfeeding practices could prevent more than 800 000 deaths in children under 5 and 20 000 deaths in women due to breast cancer annually [3]. In contrast, not breastfeeding is associated with annual economic losses of around 302 billion US dollar and lower intelligence [4].
- This study focuses on the Republic of Indonesia, the largest country in Southeast Asia, where breastfeeding is a norm; almost all children are breastfed [5]. However, 24 children out of 1000 live births die before their fifth birthday, and 32% of children are stunted in Indonesia [6]. As the prevalence of wasting and underweight is 10.2% and 17.7%, respectively, child undernutrition remains a public health problem that cannot be ignored [6]. Breastfeeding is among the best-buy interventions to tackle child malnutrition [7].
- Indonesia is on course to achieve the World Health Assembly target by 2025, with half of all infants receiving EBF [8]. However, more efforts are needed to reach the national target of 60% by 2024 [9]. Similarly, EIBF practice has moderately improved over time, with the latest figure of 57% [6]. Therefore, more effective interventions are pivotal to achieving the global target of 70% [1]. In 2018, 77% of mothers in Indonesia practiced continued breastfeeding at 1 year (CBF-1); as for continued breastfeeding at 2 years (CBF-2), the prevalence was 55% [10]. No national targets have been set for CBF-1 and CBF-2, but it is clear that more strategic interventions targeting both breastfeeding practices are required to reach the global targets of 80% and 60%, respectively [1]. To formulate effective interventions improving breastfeeding practices, understanding the barriers and facilitators of each breastfeeding indicator is crucial, as a mother’s decision to breastfeeding is determined by numerous factors that can vary between and within nationalities [4]. Therefore, this study aimed to analyze factors associated with optimal breastfeeding practices in Indonesia.
INTRODUCTION
- Data Source
- This report presents secondary data analyses from the 2017 Indonesia Demographic and Health Survey (IDHS), a nationally representative survey conducted in all 34 provinces of the country.
- Sample Size and Sampling
- The IDHS employed 2-stage stratified sampling using census blocks as the primary sampling unit and stratification by rural and urban areas in each province. Census blocks were selected using systematic random sampling proportional to size. In the second stage, 25 households were chosen from each census block selected. Details on the sampling procedure and survey methodology can be found elsewhere [10].
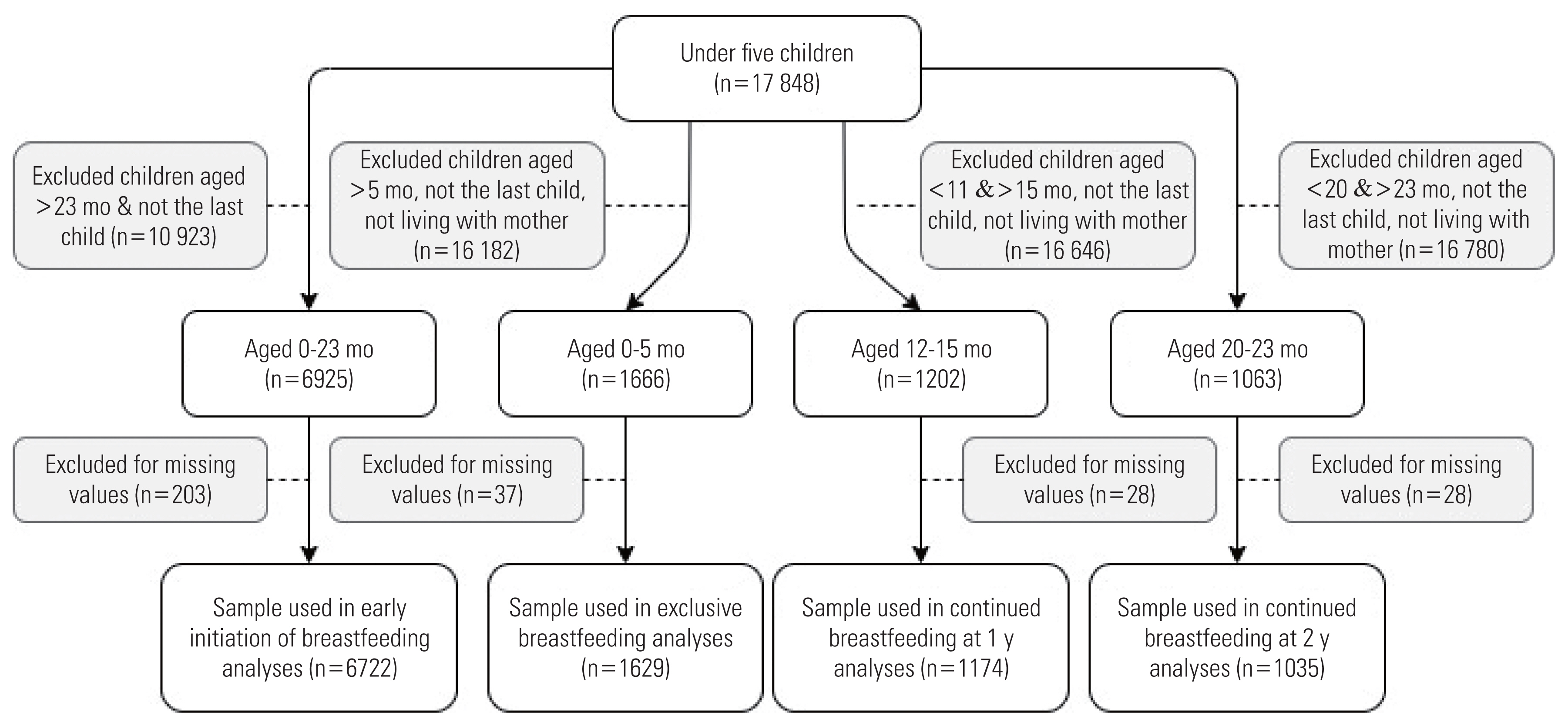
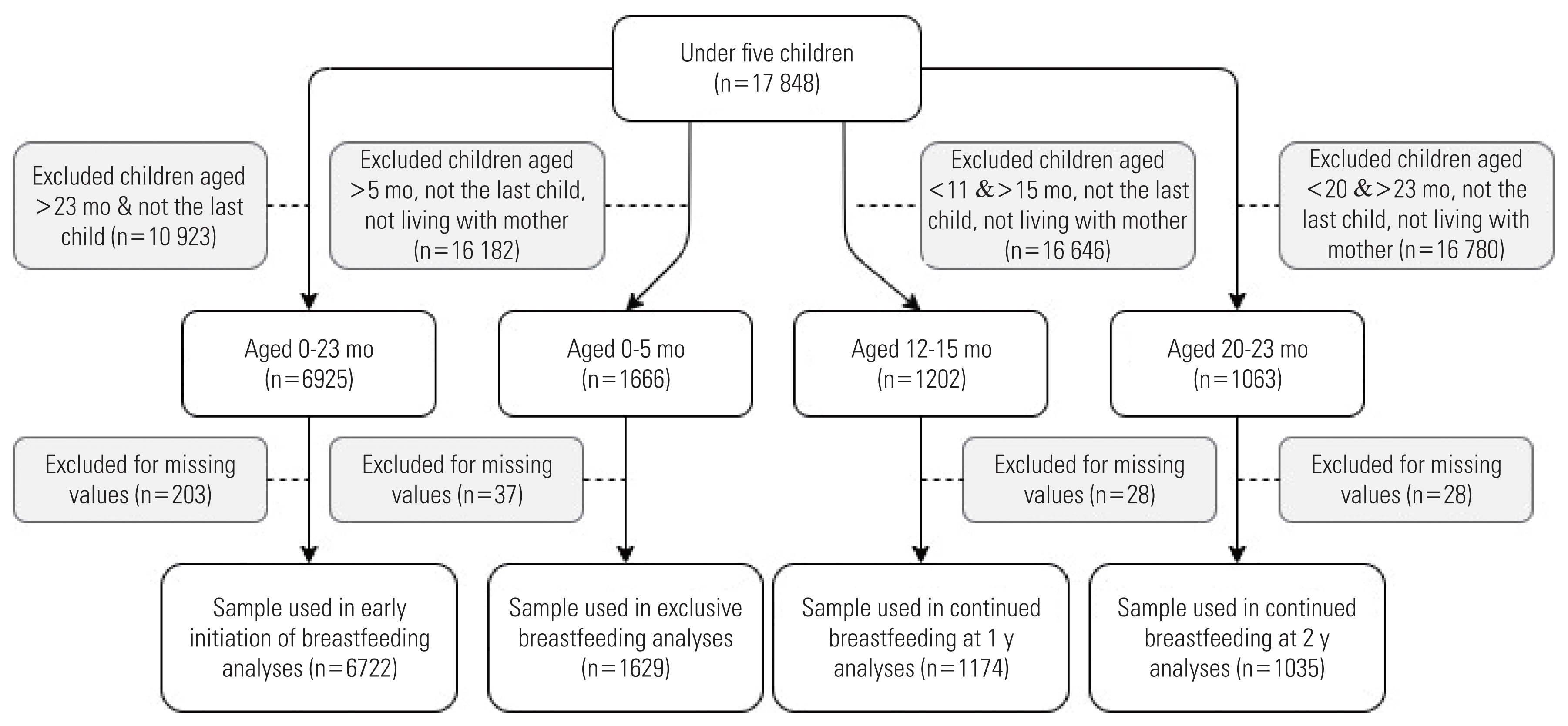
- The outcomes (i.e., EIBF, EBF, CBF-1, and CBF-2) were analyzed using different samples. The inclusion criteria were last-born infants aged within the WHO guidelines for each outcome of interest that had no missing data on the variables included in the analyses. Infants who were either deceased or did not live with their mother were excluded from the analyses of EBF, CBF-1, and CBF-2. The total samples for EIBF, EBF, CBF-1, and CBF-2 were 6722, 1629, 1174, and 1035, respectively.
- Figure 1 illustrates the details of the sample selection procedures for each outcome.
- Study Variables
- The outcomes analyzed were: (1) EIBF: defined as the proportion of last-born infants aged below 24 months who were put to their mother’s breast within 1 hour of birth; (2) EBF: the proportion of infants aged 0–5 months who were fed breastmilk (expressed milk or from a wet nurse) only, except for oral rehydration salt, vitamins, minerals, and medicines in the previous day; (3) CBF-1: the percentage of infants aged 12–15 months receiving breastmilk during the previous day; and (4) CBF-2: the proportion of children aged 20–23 months given breastmilk during the previous day [10,11].
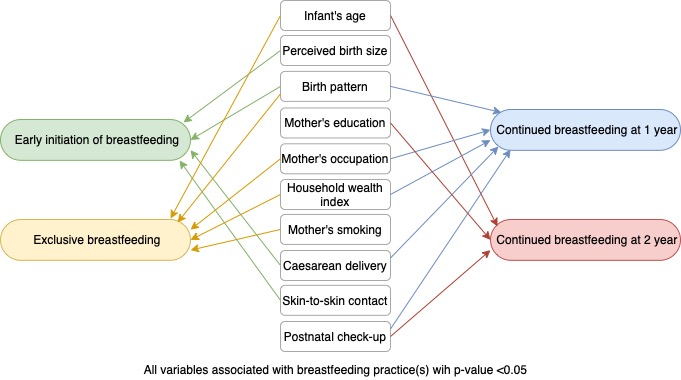
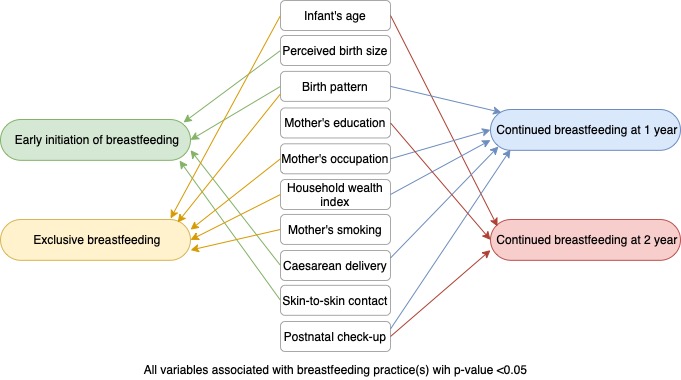
- Independent variables included bio-demographic, socioeconomic, and behavioral characteristics selected based on the existing framework (Figure 2) and previous studies [4,12,13]. Bio-demographic covariates consisted of the infant’s age and sex (male/female), the mother’s age at delivery (≤19, 20–34, or ≥35 years), infant’s birth size as perceived by the mother (small, average, or large), birth pattern (first child, not first-child with a birth interval <2 years, or not first-child with a birth interval ≥2 years), and place of residence (urban or rural). Socioeconomic factors included the mother’s education (primary or no formal education, secondary, or college or higher), the mother’s occupation (not working, agriculture, or others), and the household wealth index. The household wealth index was formed based on household’s assets, house materials, and access to water and sanitation; this index was divided into terciles (poor, middle, or rich). Behavioral characteristics included the mother’s smoking status (yes or no), antenatal care (ANC) visits (none, 1–3, or 4 times or more), type of delivery (cesarean or vaginal), place of delivery (health facility or non-health facility), skin-to-skin contact (yes or no), time of first postnatal check-up (no visit, <24 hours, 1–6 days, or 1 week or more).
- Statistical Analysis
- Descriptive statistics were used to present counts and percentages, except for the infant’s age, which was presented as mean and standard deviation. We constructed multivariate logistic regression models to identify factors associated with the outcomes, which were reported as adjusted odds ratios (aORs) with 95% confidence intervals (CIs). All variables considered to be conceptually relevant were included in the full models regardless of the results of the initial analyses. Independent covariates were excluded stepwise from the full model, starting with a covariate with the highest p-value. The decision to keep a variable in the model was based on its relevancy, the change in the odds ratio, and the p-value. To check multicollinearity in the final models, we ran a simple correlation matrix and calculated the variance inflation factor. The models were also assessed using the Archer-Lemeshow goodness-of-fit test. Both the descriptive and regression analyses considered the study design and multi-stage sampling procedure using the svyset command. All analyses were conducted using Stata version 15.1 (StataCorp., College Station, TX, USA). The results of the crude analyses and the full models can be found in the Supplemental Material 1.
- Ethics Statement
- We obtained access to the dataset after registering the study (authorization letter No. 151332). Ethical clearance was the responsibility of the institution conducting the survey.
METHODS
- The average age of the EIBF sample was 11.6 months; as for EBF, CBF-1, and CBF-2, the averages ages were 2.8 months, 13.6 months, and 21.0 months, respectively (Table 1). Overall, the distribution of the respondent characteristics in percentages was similar across the outcomes. Most infants were born to mothers aged 20–34 years old, and just under 60% were perceived as having an average birth size. First-born infants accounted for approximately one-third of the respondents. The number of respondents residing in rural areas was almost equal to those in urban areas. Over half (57.5–60.0%) of mothers had finished secondary school, and more than half were housewives. The proportions of respondents coming from poor, middle, and rich families were almost evenly distributed. The percentage of smoking mothers ranged from 0.9% to 2.2%, and almost all mothers received 4 or more ANC visits and delivered at a health facility. Around 60% of the infants experienced skin-to-skin contact, and about 30% of mothers did not receive a postnatal visit.
- Table 2 presents the final model for the determinants of EIBF, EBF, CBF-1, and CBF-2. Small perceived birth size, first child, and cesarean delivery were barriers to EIBF, while skin-to-skin contact increased the odds of EIBF (aOR, 2.17; 95% CI, 2.23 to 2.89). Older children were less likely to receive EBF. As with EIBF, being a first-born child was also a barrier to EBF. The other barriers were mothers working outside the agricultural sector, middle household wealth index, and smoking mothers.
- Infants’ age was among the determinants of CBF-2; a 1-month increase in the infant’s age was associated with a 15% decrease in the odds of CBF-2 (aOR, 0.85; 95% CI, 0.74 to 0.99); however, this did not affect CBF-1. A higher level of maternal education was negatively associated with CBF-2. In contrast, this variable had no significant association in the model for CBF-1. The last identified determinant of CBF-2 was the time of the first postnatal visit, which was also among the facilitators of CBF-1. The other factors associated with CBF-1 were a non-first child with a birth interval ≥2 years, a low-income family, mothers with other occupations, and vaginal delivery.
RESULTS
- Our findings revealed that perceived birth size, birth pattern, type of delivery, and skin-to-skin contact were associated with EIBF. EBF was influenced by the infant’s age, birth pattern, mother’s occupation, wealth index, and maternal smoking. Postnatal care improved the likelihood of both CBF-1 and CBF-2. Birth pattern, the mother’s occupation, and wealth index were also among the determinants of CBF-1. The other determinants of CBF-2 included the mother’s education and infant’s age.
- The present analysis showed that older children had lower odds of EBF and CBF-2. A large body of evidence has also confirmed that younger children are more likely to be exclusively breastfed than older ones [12,14,15]. A possible explanation is mothers’ perception that giving only breastmilk is insufficient as infants grow larger [16]. Therefore, educational messages and supports clarifying this misperception could be among the key strategies to improve EBF practice. It is important to note that mothers of older infants should be given more attention. Consistent with this study, previous analyses also found that the infant’s age was negatively associated with CBF-2 [12]. A reason could be the rapid growth of formula feeding practice, and giving formula to infants at any age decreased the odds of breastfeeding at 2 years [17,18].
- In line with previous studies, infants perceived as small were less likely to receive EIBF [12,15,19]. Small birth size as perceived by the mothers may indicate that the infants had low birth weight, which was a risk factor of EIBF [20]. Small newborns are more likely to be physically immature and lack coordination in suckling, respiratory system, and reflexes, which could delay the infant’s ability to start breastfeeding within 1 hour [21]. Nevertheless, this study found that the infant’s birth weight did not affect EBF and continued breastfeeding practices. This finding, which corresponds to previous studies, allows mothers to achieve EBF and continued breastfeeding although their infants were smaller than average [12,20].
- This study showed that being a first child was negatively associated with EIBF, EBF, and CBF-1. Similarly, studies in South Asia and West Africa also reported that the birth interval and birth order were associated with EIBF [19,22]. Findings from studies in Ethiopia and India also concluded that birth order and birth interval were among the predictors of EBF [23,24]. First-time mothers are less likely to use healthcare services regarding delivery and breastfeeding; therefore, the prevalence of EIBF and EBF was lower in this group. Another explanation could be their inexperience in breastfeeding, making them lack confidence in following the breastfeeding recommendations.
- Inconsistent findings have been reported regarding the association between maternal education and breastfeeding practices. Evidence from this analysis and studies in Sri Lanka and Bangladesh showed that maternal education was associated with neither EIBF nor EBF [20,25]. Even so, that variable was negatively associated with CBF-2; mothers with the highest education level had the lowest odds of CBF-2. This finding is consistent with a study in India [26]. Generally, highly educated mothers in low-to-middle income countries (LMICs) are less likely to breastfeed; they tend to opt for breastmilk substitutes due to their increased purchasing power. It is also possible that poorer mothers will switch to substitutes as their income increases [3].
- EBF and CBF-1 were less common among mothers working outside the agricultural sector than non-working mothers, in line with findings from studies in LMICs [27]. A cohort study in Australia also showed that mothers going back to work by 1 year after delivery had lower odds of CBF-1 than those not returning to work within that period [18]; an explanation could relate to the mother’s workload and the short duration maternity leave. Similar to maternal employment, the household wealth index also affected both EBF and CBF-1; mothers from poorer families were more likely to follow EBF and CBF-1 recommendations, consistent with a Nepalese study for EBF [12]. In LMICs, breastfeeding was found to be more common among mothers from a lower economic background [3]; since they may have had no alternatives for feeding their infants. In contrast, mothers from wealthier families can buy breastmilk substitutes if they do not receive the proper knowledge and other breastfeeding-supporting conditions.
- A review concluded that smoking was one of the strongest determinants of early breastfeeding practices in developed countries [28]. Consistent with that finding, smoking was associated with EBF in the present analysis. Health promotion efforts need to raise awareness on the adverse effects of smoking, particularly during breastfeeding, as the nicotine concentration in breastmilk is 3 times higher than in the plasma in smoking women. Smoking also reduces breastmilk’s protective properties, volume, and duration of breastfeeding [29].
- Corresponding to the results in this study, cesarean delivery has been consistently seen as a barrier to EIBF [12,19,25]. A plausible explanation for this association is the physical pain experienced by the mothers after delivering via cesarean section, which makes them feel uncomfortable in adapting to breastfeeding positions [30]. It is also possible that mothers take time to recover from anesthesia, and their newborns could also be in respiratory distress, resulting in them being placed in a different room from their mother [31]. In some hospitals in Indonesia, post-cesarean delivery procedures prevent infant-mother contact since both are taken to a separate room [32]. Antibiotics administered to mothers after cesarean sections may also delay breastfeeding initiation as mothers do not want antibiotics to pass to their infants through breastmilk [33].
- Findings on the impact of cesarean delivery on EBF or CBF are inconsistent. Some previous studies reported that cesarean section was negatively associated with both practices [33,34]. In other studies, including this present analysis, the type of delivery was not among the determinants of EBF [12]. Nevertheless, given that the number of mothers delivering via cesarean section is increasing, extra attention for this at-risk group should be of priority [35]. For example, breastfeeding education and support could be delivered in the early postnatal period, and steps could be taken to promote immediate skin-to-skin contact [36]. A systematic review on skin-to-skin contact concluded that this practice plays an essential role in successful breastfeeding until 4 months [37]. In other words, the impact of skin-to-skin contact may not last throughout the entire period of EBF, similar to the present analysis of EBF, which found no significant association between skin-to-skin contact and EBF.
- To date, findings on the association between postnatal visits and breastfeeding are inconclusive. In Bangladesh and India, postnatal visits encouraged EIBF [12,25], while in Afghanistan and Pakistan, they delayed breastfeeding initiation [12]. An analysis of the Myanmar Demographic and Health Survey reported that mothers who received postnatal check-ups were less likely to practice EBF [15]. In the present report, postnatal visits had no impact on either EIBF or EBF. Regarding EBF, the current finding is consistent with the evidence from South Asia [12]. Our study also found that mothers receiving the first postnatal visit after 6 days were more likely to breastfeed their infants at 1 year and 2 years than those who never had postnatal visits. This finding can be explained by a previous analysis showing that breastfeeding counseling, as a part of postnatal care activities, improved mothers’ breastfeeding self-efficacy, which promoted optimal breastfeeding practices in Indonesia [38]. In addition, postnatal visits may ensure that mothers receive ongoing support to overcome barriers during breastfeeding, especially for those undergoing cesarean delivery [38]. However, a previous report found that postnatal visits did not impact CBF-1 [12]. This inconsistency is probably due to the wide variety in postnatal care quality across regions.
- In Indonesia, living with the extended family may influence breastfeeding practices since family members, particularly the husband, mother, mother-in-law, and grandmother, play critical roles in supporting mothers to breastfeed their infants [38,39]. However, family members from older generations (e.g., grandmothers) usually lack support for EBF, as they often advise mothers to give newborns or under 6-month infants substances other than breastmilk [40]. At the same time, support in following the recommended breastfeeding practices could also come from family members [39]. Therefore, prenatal and postnatal breastfeeding education targeting mothers and other family members is essential to improve family members’ awareness of the importance of optimal breastfeeding practices [38].
- A notable strength of this study is the use of nationally representative data with an internationally standardized methodology and a validated questionnaire, enabling generalizability to Indonesian females. The definitions of the variables included were based on international guidelines, making the results comparable across countries. Another strength is that it included CBF-1 and CBF-2 indicators, which are often overlooked, in addition to EBF and EIBF. As each indicator had differing determinants, analyzing all these indicators provides additional insights. Nonetheless, the survey design also comes with limitations. EIBF, as a variable generated from a recall-based interview, might introduce bias. Events that happened a long time in the past, such as ANC visits and skin-to-skin contact, were also at risk of memory bias. However, this can be assumed to only occur on a small scale because pregnancy and delivery are special events for mothers. In addition, the use of a large dataset would mitigate the risk of bias.
- Improving breastfeeding practices is critical for preventing child malnutrition, morbidity, and mortality in LMICs. This study outlined a preliminary impression of the facilitators and barriers of optimal breastfeeding practices in Indonesia, which can bring meaningful insights for designing breastfeeding interventions. A general overview of the findings revealed no conflicting conclusions among the identified determinants in this study; no factor acted as a barrier for an outcome but became a facilitator for another one. This consistency is essential for policies and programs targeting multiple indicators of optimal breastfeeding practices to achieve maximum benefits.
DISCUSSION
SUPPLEMENTAL MATERIALS
Supplemental Material 1.
ACKNOWLEDGEMENTS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
This work was financially supported by Universitas Muhammadiyah Surakarta (grant No. 333.4/A.3-III/FIK/1/2021).
-
AUTHOR CONTRIBUTIONS
Conceptualization: Nurokhmah S, Rahmawaty S. Data curation: Nurokhmah S. Formal analysis: Nurokhmah S. Funding acquisition: Nurokhmah S, Rahmawaty S, Puspitasari DI. Methodology: Nurokhmah S, Rahmawaty S. Project administration: Puspitasari DI. Visualization: Nurokhmah S. Writing – original draft: Nurokhmah S. Writing – review & editing: Nurokhmah S, Rahmawaty S, Puspitasari DI.
Notes
| Characteristics | EIBF (n=6722) | EBF (n=1629) | CBF-1 (n=1174) | CBF-2 (n=1015) |
|---|---|---|---|---|
| Bio-demographic variables | ||||
| Infant’s age, mean±SD (mo) | 11.6±6.7 | 2.8±1.6 | 13.6±1.1 | 21.0±1.1 |
| Sex of the infant | ||||
| Male | 3504 (51.7) | 800 (50.3) | 610 (49.7) | 532 (51.9) |
| Female | 3218 (48.3) | 829 (49.7) | 564 (50.3) | 503 (48.1) |
| Mother’s age at delivery (y) | ||||
| ≤19 | 626 (9.0) | 161 (9.8) | 120 (9.8) | 80 (7.8) |
| 20–34 | 4947 (73.7) | 1221 (75.4) | 860 (73.2) | 763 (73.9) |
| ≥35 | 1149 (17.2) | 247 (14.8) | 193 (17.0) | 192 (18.3) |
| Perceived birth size | ||||
| Small | 884 (11.8) | 204 (11.4) | 159 (11.3) | 146 (12.5) |
| Average | 3620 (57.2) | 888 (56.3) | 616 (58.1) | 556 (59.2) |
| Large | 2218 (31.0) | 537 (32.2) | 399 (30.7) | 333 (28.3) |
| Birth pattern | ||||
| First child | 2161 (33.0) | 504 (31.0) | 398 (34.9) | 330 (33.1) |
| Not first-child, birth interval <2 y | 460 (5.6) | 127 (6.9) | 78 (5.6) | 55 (4.5) |
| Not first-child, birth interval ≥2 y | 4101 (61.4) | 998 (62.1) | 698 (59.4) | 650 (62.4) |
| Place of residence | ||||
| Urban | 3326 (49.1) | 758 (47.1) | 591 (49.5) | 524 (50.2) |
| Rural | 3396 (50.9) | 871 (52.9) | 583 (50.5) | 511 (49.8) |
|
|
||||
| Socioeconomic variables | ||||
| Mother’s education | ||||
| Primary or no formal education | 1557 (23.8) | 382 (24.0) | 284 (25.2) | 227 (22.5) |
| Secondary | 3835 (59.3) | 939 (60.0) | 650 (57.5) | 592 (59.5) |
| College or higher | 1330 (16.9) | 308 (15.9) | 240 (17.2) | 216 (18.0) |
| Mother’s occupation | ||||
| Not working | 3599 (55.9) | 941 (58.8) | 655 (59.4) | 517 (52.4) |
| Agriculture | 550 (6.8) | 111 (5.6) | 97 (7.0) | 85 (8.1) |
| Others1 | 2573 (37.3) | 577 (35.6) | 422 (33.6) | 433 (39.5) |
| Household wealth index | ||||
| Poor | 2689 (33.3) | 686 (34.3) | 469 (34.6) | 400 (32.5) |
| Middle | 2069 (33.3) | 506 (35.0) | 361 (32.1) | 299 (30.7) |
| Rich | 1964 (33.3) | 437 (30.7) | 344 (33.3) | 336 (36.8) |
|
|
||||
| Behavioral characteristics | ||||
| Mother’s smoking status | ||||
| Yes | 127 (1.4) | 23 (1.2) | 20 (0.9) | 24 (2.2) |
| No | 6595 (98.6) | 1606 (98.8) | 1154 (99.1) | 1011 (97.8) |
| Antenatal care visits (times) | ||||
| None | 186 (2.3) | 48 (2.8) | 37 (2.7) | 23 (1.7) |
| 1–3 | 592 (7.0) | 174 (8.9) | 101 (6.4) | 80 (5.8) |
| ≥4 | 5944 (90.6) | 1407 (88.3) | 1036 (90.9) | 932 (92.5) |
| Cesarean delivery | ||||
| Yes | 1230 (18.9) | 288 (18.1) | 206 (18.2) | 194 (19.1) |
| No | 5492 (81.1) | 1341 (81.9) | 968 (81.8) | 841 (80.9) |
| Place of delivery | ||||
| Health facility | 5313 (84.1) | 1311 (85.5) | 925 (84.3) | 814 (83.5) |
| Non-health facility | 1409 (15.9) | 318 (14.5) | 249 (15.7) | 221 (16.5) |
| Skin-to-skin contact | ||||
| Yes | 3744 (60.3) | 876 (59.0) | 670 (61.9) | 581 (60.3) |
| No | 2948 (39.7) | 753 (41.0) | 504 (38.1) | 454 (39.7) |
| Time of first postnatal visit | ||||
| No visit | 2400 (33.3) | 651 (37.3) | 378 (30.0) | 380 (35.0) |
| <24 hr | 573 (6.7) | 128 (6.4) | 100 (6.5) | 94 (8.3) |
| 1–6 day | 1734 (28.5) | 437 (29.5) | 312 (30.0) | 258 (26.4) |
| ≥1 wk | 2015 (31.4) | 413 (26.8) | 384 (33.5) | 303 (30.2) |
| Variables | EIBF | EBF | CBF-1 | CBF-2 |
|---|---|---|---|---|
| Infant’s age (mo)2,3 | 0.97 (0.83, 1.33) | 0.85 (0.74, 0.99)* | ||
| 0–1 | - | 1.00 (reference) | - | - |
| 2–3 | - | 0.58 (0.42, 0.79)*** | - | - |
| 4–5 | - | 0.31 (0.22, 0.42)*** | - | - |
|
|
||||
| Perceived birth size | ||||
| Small | 0.71 (0.58, 0.85)** | - | - | - |
| Average | 1.00 (reference) | - | - | - |
| Large | 0.97 (0.84, 1.13) | - | - | - |
|
|
||||
| Birth pattern | ||||
| First child | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | - |
| Not first-child, birth interval <2 y | 1.42 (1.09, 1.85)** | 1.69 (1.01, 2.82)* | 1.60 (0.76, 3.38) | - |
| Not first-child, birth interval ≥2 y | 1.43 (1.25, 1.63)*** | 1.28 (0.97, 1.70) | 1.87 (1.30, 2.68)** | - |
|
|
||||
| Mother’s education | ||||
| No education or primary | - | - | 1.00 (reference) | 1.00 (reference) |
| Secondary | - | - | 0.74 (0.44, 1.25) | 0.68 (0.45, 1.04) |
| College or higher | - | - | 0.70 (0.36, 1.34) | 0.45 (0.26, 0.77)** |
|
|
||||
| Mother’s occupation | ||||
| Not working | - | 1.00 (reference) | 1.00 (reference) | - |
| Agriculture | - | 0.81 (0.48, 1.35) | 1.26 (0.54, 2.96) | - |
| Others4 | - | 0.65 (0.49, 0.86)** | 0.68 (0.47, 0.99)* | - |
|
|
||||
| Household wealth index | ||||
| Poor | - | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Middle | - | 0.67 (0.49, 0.91)* | 0.52 (0.34, 0.78)** | 1.12 (0.77, 1.65) |
| Rich | - | 0.75 (0.53, 1.06) | 0.34 (0.21, 0.56)*** | 0.75 (0.51, 1.11) |
|
|
||||
| Mother’s smoking status | ||||
| Yes | - | 1.00 (reference) | - | - |
| No | - | 2.97 (1.12, 7.89)* | - | - |
|
|
||||
| Antenatal care visit (times) | ||||
| None | - | - | 1.00 (reference) | - |
| 1–3 | - | - | 1.34 (0.35, 5.12) | - |
| ≥4 | - | - | 2.67 (0.80, 8.92) | - |
|
|
||||
| Cesarean delivery | ||||
| Yes | 1.00 (reference) | - | 1.00 (reference) | - |
| No | 2.17 (1.83, 2.57)*** | - | 1.76 (1.15, 2.68)** | - |
|
|
||||
| Place of delivery | ||||
| Health facility | - | - | 1.00 (reference) | - |
| Non-health facility | - | - | 0.73 (0.42, 1.26) | - |
|
|
||||
| Skin-to-skin contact | ||||
| Yes | 2.17 (2.23, 2.89)*** | - | - | - |
| No | 1.00 (reference) | - | - | - |
|
|
||||
| Time of first postnatal visit | ||||
| No visit | - | - | 1.00 (reference) | 1.00 (reference) |
| <24 hr | - | - | 1.12 (0.52, 2.38) | 1.36 (0.75, 2.47) |
| 1–6 day | - | - | 1.42 (0.90, 2.23) | 1.06 (0.72, 1.56) |
| ≥1 wk | - | - | 1.53 (1.03, 2.28)* | 1.47 (1.00, 2.15)* |
Values are presented as adjusted odds ratio (95% confidence interval).
The final model for EIBF included perceived birth size, birth pattern, type of delivery, and skin-to-skin contact (Archer-Lemeshow goodness-of-fit 0.689); The final model for EBF included infant’s age, birth pattern, mother’s occupation, household wealth index, and mother’s smoking status (Archer-Lemeshow goodness-of-fit 0.508); The final model for CBF-1 included infant’s age, birth pattern, mother’s education, mother’s occupation, household wealth index, antenatal care visits, type of delivery, place of delivery, and time of the first postnatal visit (Archer-Lemeshow goodness-of-fit 0.660); The final model for CBF-2 included infant’s age, mother’s education, household wealth index, and time of the first postnatal visit (Archer-Lemeshow goodness-of-fit 0.883).
EIBF, early initiation of breastfeeding; EBF, exclusive breastfeeding; CBF-1, continued breastfeeding at 1 year; CBF-2, continued breastfeeding at 2 years.
1 The sample sizes of each group are different since models differed by outcome (see Figure 1).
2 Age was not included as a variable in the analyses of EIBF.
3 Fitted as a continuous variable in the analyses of CBF-1 (values: 12, 13, 14, and 15 months) and CBF-2 (values: 20, 21, 22, and 23 months) so that the adjusted odds ratio reflects the effect of a 1-month increase in age.
4 Included those worked as a professional, technician, manager, clerical, sales, and industrial worker.
* p<0.05,
** p<0.01,
*** p<0.001.
- 1. World Health Organization. Global breastfeeding scorecard 2017 tracking progress for breastfeeding policies and programmes; 2017 [cited 2021 Apr 18]. Available from: https://www.who.int/publications/m/item/global-breastfeeding-scorecard-2017-tracking-progress-for-breastfeeding-policies-and-programmes
- 2. World Health Organization. Global strategy for infant and young child feeding; 2003 [cited 2021 Apr 18]. Available from: https://www.who.int/publications/i/item/9241562218
- 3. Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387(10017):475-490ArticlePubMed
- 4. Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016;387(10017):491-504ArticlePubMed
- 5. United Nations International Children’s Emergency Fund. The state of the world’s children 2019 Children, food and nutrition: growing well in a changing world; [cited 2021 Apr 19]. Available from: https://www.unicef.org/reports/state-of-worlds-children-2019
- 6. National Institute of Health. Research and Development. Report on the 2018; National Basic Health Research; 2018 [cited 2021 Apr 18]. Available from: http://labdata.litbang.kemkes.go.id/images/download/laporan/RKD/2018/Laporan_Nasional_RKD2018_FINAL.pdf . (Indonesian)
- 7. Shekar M, Kakietek J, Dayton Eberwein J, Walters D. An investment framework for nutrition: reaching the global targets for stunting, anemia, breastfeeding, and wasting; 2017 [cited 2021 Apr 20]. Available from: https://openknowledge.worldbank.org/handle/10986/26069
- 8. Development Initiatives. 2020 Global nutrition report; [cited 2021 Apr 19]. Available from: https://globalnutritionreport.org/reports/2020-global-nutrition-report/
- 9. Kementrian Kesehatan Republic Indonesia. Regulation of the Minister of Health of the Republic of Indonesia no 21 year 2020 on the 2020–2024 strategic plan of the Ministry of Health; [cited 2021 Dec 2]. Available from: https://peraturan.bpk.go.id/Home/Details/152564/permenkes-no-21-tahun-2020 (Indonesian)
- 10. National Population and Family Planning Board (BKKBN), Statistics Indonesia (BPS), Ministry of Health (Kemenkes), ICF. Indonesia Demographic and Health Survey 2017; 2018 [cited 2021 Apr 18]. Available from: https://dhsprogram.com/pubs/pdf/FR342/FR342.pdf
- 11. World Health Organization. Indicators for assessing infant and young child feeding practices: definitions and measurement methods; [cited 2021 Apr 18]. Available from: https://www.who.int/publications/i/item/9789240018389
- 12. Benedict RK, Craig HC, Torlesse H, Stoltzfus RJ. Trends and predictors of optimal breastfeeding among children 0–23 months, South Asia: analysis of national survey data. Matern Child Nutr 2018;14(Suppl 4):e12698ArticlePubMedPMC
- 13. United Nations. International Children’s Emergency Fund. From the first hour of life: a new report on infant and young child feeding; [cited 2021 Apr 19]. Available from: https://data.unicef.org/resources/first-hour-life-new-report-breastfeeding-practices/
- 14. Nkoka O, Ntenda PA, Kanje V, Milanzi EB, Arora A. Determinants of timely initiation of breast milk and exclusive breastfeeding in Malawi: a population-based cross-sectional study. Int Breastfeed J 2019;14: 37ArticlePubMedPMC
- 15. Yadanar , Mya KS, Witvorapong N. Determinants of breastfeeding practices in Myanmar: results from the latest nationally representative survey. PLoS One 2020;15(9):e0239515ArticlePubMedPMC
- 16. Afiyanti Y, Juliastuti D. Exclusive breastfeeding practice in Indonesia. Br J Midwifery 2012;20(7):484-491Article
- 17. Baker P, Smith J, Salmon L, Friel S, Kent G, Iellamo A, et al. Global trends and patterns of commercial milk-based formula sales: is an unprecedented infant and young child feeding transition underway? Public Health Nutr 2016;19(14):2540-2550ArticlePubMedPMC
- 18. Scott J, Ahwong E, Devenish G, Ha D, Do L. Determinants of continued breastfeeding at 12 and 24 months: results of an Australian cohort study. Int J Environ Res Public Health 2019;16(20):3980ArticlePubMedPMC
- 19. Ezeh OK, Ogbo FA, Stevens GJ, Tannous WK, Uchechukwu OL, Ghimire PR, et al. Factors associated with the early initiation of breastfeeding in economic community of West African States (ECOWAS). Nutrients 2019;11(11):2765ArticlePubMedPMC
- 20. Senarath U, Siriwardena I, Godakandage SS, Jayawickrama H, Fernando DN, Dibley MJ. Determinants of breastfeeding practices: an analysis of the Sri Lanka Demographic and Health Survey 2006–2007. Matern Child Nutr 2012;8(3):315-329ArticlePubMed
- 21. Adhikari M, Khanal V, Karkee R, Gavidia T. Factors associated with early initiation of breastfeeding among Nepalese mothers: further analysis of Nepal Demographic and Health Survey, 2011. Int Breastfeed J 2014;9(1):21ArticlePubMedPMC
- 22. Sharma IK, Byrne A. Early initiation of breastfeeding: a systematic literature review of factors and barriers in South Asia. Int Breastfeed J 2016;11: 17ArticlePubMedPMC
- 23. Tsegaw SA, Ali Dawed Y, Tadesse Amsalu E. Exploring the determinants of exclusive breastfeeding among infants under-six months in Ethiopia using multilevel analysis. PLoS One 2021;16(1):e0245034ArticlePubMedPMC
- 24. Dhami MV, Ogbo FA, Akombi-Inyang BJ, Torome R, Agho KE. on behalf of the Global Maternal and Child Health Research Collaboration (GloMACH). Understanding the enablers and barriers to appropriate infants and young child feeding practices in India: a systematic review. Nutrients 2021;13(3):825ArticlePubMedPMC
- 25. Karim F, Khan AN, Tasnim F, Chowdhury MA, Billah SM, Karim T, et al. Prevalence and determinants of initiation of breastfeeding within one hour of birth: an analysis of the Bangladesh Demographic and Health Survey, 2014. PLoS One 2019;14(7):e0220224ArticlePubMedPMC
- 26. Oakley L, Baker CP, Addanki S, Gupta V, Walia GK, Aggarwal A, et al. Is increasing urbanicity associated with changes in breastfeeding duration in rural India? An analysis of cross-sectional household data from the Andhra Pradesh children and parents study. BMJ Open 2017;7(9):e016331ArticlePubMedPMC
- 27. Kavle JA, LaCroix E, Dau H, Engmann C. Addressing barriers to exclusive breast-feeding in low- and middle-income countries: a systematic review and programmatic implications. Public Health Nutr 2017;20(17):3120-3134ArticlePubMedPMC
- 28. Cohen SS, Alexander DD, Krebs NF, Young BE, Cabana MD, Erdmann P, et al. Factors associated with breastfeeding initiation and continuation: a meta-analysis. J Pediatr 2018;203: 190-196ArticlePubMed
- 29. Napierala M, Mazela J, Merritt TA, Florek E. Tobacco smoking and breastfeeding: effect on the lactation process, breast milk composition and infant development. A critical review. Environ Res 2016;151: 321-338ArticlePubMed
- 30. Saeed G, Fakhar S, Imran T, Khawaja Abbas L. The effect of modes of delivery on infants’ feeding practices. Iran J Med Sci 2011;36(2):128-132PubMedPMC
- 31. Liben ML, Yesuf EM. Determinants of early initiation of breastfeeding in Amibara district, Northeastern Ethiopia: a community based cross-sectional study. Int Breastfeed J 2016;11: 7ArticlePubMedPMC
- 32. Titaley CR, Loh PC, Prasetyo S, Ariawan I, Shankar AH. Socio-economic factors and use of maternal health services are associated with delayed initiation and non-exclusive breastfeeding in Indonesia: secondary analysis of Indonesia Demographic and Health Surveys 2002/2003 and 2007. Asia Pac J Clin Nutr 2014;23(1):91-104PubMed
- 33. Hoang Nguyen PT, Binns CW, Vo Van Ha A, Nguyen CL, Khac Chu T, Duong DV, et al. Caesarean delivery associated with adverse breastfeeding practices: a prospective cohort study. J Obstet Gynaecol 2020;40(5):644-648ArticlePubMed
- 34. Zhao J, Zhao Y, Du M, Binns CW, Lee AH. Does caesarean section affect breastfeeding practices in China? A systematic review and meta-analysis. Matern Child Health J 2017;21(11):2008-2024ArticlePubMed
- 35. Verma V, Vishwakarma RK, Nath DC, Khan HT, Prakash R, Abid O. Prevalence and determinants of caesarean section in South and South-East Asian women. PLoS One 2020;15(3):e0229906ArticlePubMedPMC
- 36. Beake S, Bick D, Narracott C, Chang YS. Interventions for women who have a caesarean birth to increase uptake and duration of breastfeeding: a systematic review. Matern Child Nutr 2017;13(4):e12390ArticlePubMed
- 37. Moore ER, Anderson GC, Bergman N, Dowswell T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev 2012;5(5):CD003519ArticlePubMed
- 38. Titaley CR, Dibley MJ, Ariawan I, Mu’asyaroh A, Alam A, Damayanti R, et al. Determinants of low breastfeeding self-efficacy amongst mothers of children aged less than six months: results from the BADUTA study in East Java, Indonesia. Int Breastfeed J 2021;16(1):12ArticlePubMedPMC
- 39. Ratnasari D, Paramashanti BA, Hadi H, Yugistyowati A, Astiti D, Nurhayati E. Family support and exclusive breastfeeding among Yogyakarta mothers in employment. Asia Pac J Clin Nutr 2017;26(Suppl 1):S31-S35PubMed
- 40. Susiloretni KA, Hadi H, Prabandari YS, Soenarto YS, Wilopo SA. What works to improve duration of exclusive breastfeeding: lessons from the exclusive breastfeeding promotion program in rural Indonesia. Matern Child Health J 2015;19(7):1515-1525ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Duration and Sociodemographic Factors Associated with Exclusive Breastfeeding Among Mothers in Urban and Semi-Rural Areas of Libreville and Lambaréné in Gabon
Steeve Minto'o, Fifi Claire Loembe, Midili Thècle Larissa, Mireille Mensan Pemba, Koumba Maniaga Raïssa, Mylène Mimbila-Mayi, Yolande Nzame, Essomo Murielle, Eliane Kuissi-Kamgaing, Jean Koko, Simon Ategbo
Archives of Pediatric Gastroenterology, Hepatology, and Nutrition.2024; 3(1): 1. CrossRef - Exclusive breastfeeding among Indonesian working mothers: does early initiation of breastfeeding matter?
Isyatun Mardhiyah Syahri, Agung Dwi Laksono, Maya Fitria, Nikmatur Rohmah, Masruroh Masruroh, Mara Ipa
BMC Public Health.2024;[Epub] CrossRef - Trends and determinants of early initiation of breastfeeding in Indonesia: A multivariate decomposition analysis
Siti Nurokhmah, Lucinda Middleton, Judhiastuty Februhartanty, Aryono Hendarto, Veincent Christian Pepito
PLOS ONE.2023; 18(11): e0294900. CrossRef - Association between Skin-to-Skin Contact Duration after Caesarean Section and Breastfeeding Outcomes
Juan Juan, Xiaosong Zhang, Xueyin Wang, Jun Liu, Yinli Cao, Ling Tan, Yan Gao, Yinping Qiu, Huixia Yang
Children.2022; 9(11): 1742. CrossRef
 KSPM
KSPM


 PubReader
PubReader ePub Link
ePub Link Cite
Cite