Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 50(6); 2017 > Article
-
Original Article
Mercury Exposure in Association With Decrease of Liver Function in Adults: A Longitudinal Study -
Jonghyuk Choi1
 , Sanghyuk Bae1
, Sanghyuk Bae1 , Hyungryul Lim1,2
, Hyungryul Lim1,2 , Ji-Ae Lim1
, Ji-Ae Lim1 , Yong-Han Lee1
, Yong-Han Lee1 , Mina Ha1
, Mina Ha1 , Ho-Jang Kwon1
, Ho-Jang Kwon1
-
Journal of Preventive Medicine and Public Health 2017;50(6):377-385.
DOI: https://doi.org/10.3961/jpmph.17.099
Published online: November 7, 2017
1Department of Preventive Medicine, Dankook University College of Medicine, Cheonan, Korea
2Republic of Korea Army Headquarters, Gyeryong, Korea
- Corresponding author: Mina Ha, MD, PhD 119 Dandae-ro, Dongnam-gu, Cheonan 31116, Korea Tel: +82-41-550-3854, Fax: +82-41-556-6461 E-mail: minaha@dku.edu
Copyright © 2017 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Although mercury (Hg) exposure is known to be neurotoxic in humans, its effects on liver function have been less often reported. The aim of this study was to investigate whether total Hg exposure in Korean adults was associated with elevated serum levels of the liver enzymes aspartate aminotransferase (AST), alanine transaminase (ALT), and gamma-glutamyltransferase (GGT).
-
Methods
- We repeatedly examined the levels of total Hg and liver enzymes in the blood of 508 adults during 2010-2011 and 2014-2015. Cross-sectional associations between levels of blood Hg and liver enzymes were analyzed using a generalized linear model, and nonlinear relationships were analyzed using a generalized additive mixed model. Generalized estimating equations were applied to examine longitudinal associations, considering the correlations of individuals measured repeatedly.
-
Results
- GGT increased by 11.0% (95% confidence interval [CI], 4.5 to 18.0%) in women and 8.1% (95% CI, -0.5 to 17.4%) in men per doubling of Hg levels, but AST and ALT were not significantly associated with Hg in either men or women. In women who drank more than 2 or 3 times per week, AST, ALT, and GGT levels increased by 10.6% (95% CI, 4.2 to 17.5%), 7.7% (95% CI, 1.1 to 14.7%), and 37.5% (95% CI,15.2 to 64.3%) per doubling of Hg levels, respectively, showing an interaction between blood Hg levels and drinking.
-
Conclusions
- Hg exposure was associated with an elevated serum concentration of GGT. Especially in women who were frequent drinkers, AST, ALT, and GGT showed a significant increase, with a significant synergistic effect of Hg and alcohol consumption.
- Mercury (Hg) exists naturally as elemental Hg, in inorganic mercurous and mercuric compounds, and in organic Hg compounds. These 3 types of Hg are known to have different toxicity and health effects [1,2]. Elemental and inorganic forms of Hg are predominantly absorbed through the respiratory tract, while organic Hg is mainly absorbed and bioaccumulated through the gastrointestinal tract because of its highly lipophilic nature [1]. Hg is removed from the human body through urine or feces, and the half-life of total Hg in the blood was found to be 57 days on average in a Japanese study [3].
- In the 2011-2012 National Health and Nutrition Examination Survey (NHANES) data, the geometric mean (95% confidence interval [CI]) of blood Hg concentrations in Asians was 1.93 μg/L (1.65 to 2.27 μg/L), which was the highest of all the races [4]. In the same study, levels of 2.58 μg/L were found in Chinese participants, 1.29 μg/L in Indians, and 2.48 μg/L in other Asian subgroups [4]. However, the geometric mean (95% CI) of blood Hg concentrations in the 2012 and 2015 Korean National Environmental Health Survey (KoNEHS) were 3.08 μg/L (2.96 to 3.21 μg/L) and 3.11 μg/L (3.02 to 3.21 μg/L), respectively, which were higher than the NHANES findings for Asians in 2011-2012. In the 2011 Korea National Health and Nutrition Examination Survey (KNHANES), the corresponding level was 3.08 μg/L (2.95 to 3.22 μg/L), which was lower than that of the KoNEHS in 2015, but also higher than the level of the Asians analyzed in the NHANES [5].
- The toxicity of high-level Hg exposure is well known from Minamata disease in Japan, outbreaks of which occurred in 1956 and 1965 due to the consumption of Hg-contaminated seafood [6]. In addition to neurological toxicity, high levels of Hg exposure affect various human organs, including the cardiovascular, endocrine, reproductive, and immune systems [7]. The mechanisms of its toxicity have been suggested to involve degeneration, oxidative stress, and changes in the energy metabolism of the cell, but are not fully understood [8]. The liver plays a major role in metabolism and detoxification, and Hg is thought to have structural and functional effects on the liver, but studies of the effects of Hg exposure on human liver tissue, particularly at relatively low levels, are limited.
- Previous epidemiological studies of the effects of Hg exposure on human liver function have shown inconsistent results. A previous epidemiological study showed that the Minamata area, despite being contaminated with methylmercury, did not have a higher prevalence of liver disease than other areas [9]. In a study of 320 adolescents, no significant associations were found between blood Hg levels and aspartate aminotransferase (AST) or alanine transaminase (ALT) levels [10]. Other epidemiological studies in adults and the elderly showed a significant positive association between Hg and AST, ALT, and gamma-glutamyltransferase (GGT) [11-14].
- Previous epidemiological studies have mostly analyzed cross-sectional data [10-12,14] and did not consider fish consumption as a potential confounder, although fish are a major source of Hg exposure [10,11,13,14]. In addition, no studies have examined the possibility of a nonlinear relationship between blood Hg and liver function indices, or the possible role of gender. Therefore, this study aimed to examine associations between total blood Hg concentration and levels of AST, ALT, and GGT, known risk factors for reduced liver function and non-alcoholic fatty liver disease, with stratification by gender.
INTRODUCTION
- Study Population
- The study participants were a subgroup of 2118 adults aged ≥19 years from the Korean Research Project on the Integrated Exposure Assessment of Hazardous Substances for Food Safety cohort recruited nationwide from June 3, 2010 to July 28, 2011. Subjects were sampled by a probability method based on household units stratified by 15 metropolises and provinces (Seoul, Gwangju, Busan, Incheon, Daegu, Daejeon, Ulsan, Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, and Gyeongnam), gender, age, and the sample size was allocated by the square root method [15].
- Of the 2118 participants ≥19 years old in the baseline survey, 514 were followed up from October 2, 2014 to September 12, 2015. The follow-up was carried out in 3 sequential steps: mailing a notice, sending a mobile phone text message with a request to participate, and making a telephone call to ask subjects to participate after a certain time interval. We obtained informed consent and conducted examinations at community centers or house visits. The follow-up involved administration of a questionnaire, a physical examination, 24-hour diet recall, and blood tests, the same items as in the baseline survey.
- Of the 514 followed-up participants, after excluding 6 subjects whose blood Hg levels or liver function indices (AST, ALT, GGT) were not measured in the baseline or follow-up, 508 participants were included in the analysis (Supplemental Figure 1).
- This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association (http://www.wma.net) and approved by the institutional review board of Dankook University Hospital (DKUH 2010-04-0093, DKUH 2011-03-0086, DKUH 2014-02-016, DKUH 2015-01-006).
- Blood Mercury
- To analyze blood Hg levels, 3 mL of blood was collected in an ethylenediaminetetraacetic acid tube as part of the 2010-2011 baseline survey and the 2014-2015 follow-up survey. Whole blood was stored at -80°C until analysis. The blood Hg analysis was performed using a MA-3000 direct Hg analyzer (Nippon Instrument Co., Tokyo, Japan). Quality control of the blood Hg analysis was performed intermittently using standard reference materials from the German external quality assessment scheme (Germany) and Bio-Rad (Bio-Rad Inc., Hercules, California, USA). The limit of detection was 0.20 μg/L, and no samples had a Hg level below the limit of detection.
- Liver Function Enzymes
- To analyze serum AST, ALT and GGT levels, 8 mL of blood was collected in an serum separator tube. The collected blood was stored at -80°C until analysis. The liver enzymes were analyzed using a Hitachi 7600 DDP analyzer (Hitachi High-Technologies, Tokyo, Japan).
- Covariates
- Fish intake was measured by the 24-hour diet recall method in the baseline survey and at follow-up. The 24-hour diet recall was designed to record the diet of 2 non-consecutive days, and the average intake (g/kg/d) was calculated for various food groups. Intake was classified into the first (<0.20 g/kg/d), second (0.20-0.72 g/kg/d), third (0.73-1.45 g/kg/d), and fourth quarters (≥1.46 g/kg/d) of total fish consumption. In addition, data from the questionnaire regarding gender, age (years), residence (urban or rural area), income (<100, 100-200, 200-300, 300-400, 400-600, or ≥600×104 Korean won/mo), lifetime smoking (yes or no), frequency of alcohol consumption (none, less than once a month, about once a month, 2-4 times a month, 2-3 times a week, or ≥4 times a week), and past medical history (yes or no) of hypertension (HTN), diabetes mellitus (DM), kidney disease, tuberculosis (TB), dyslipidemia, or hepatitis B were included as covariates. For the residence variable, we divided regions into 2 groups: urban (Seoul, Ulsan, Incheon, Daejeon, Gwangju, Daegu, and Busan) and rural (all others).
- Statistical Analysis
- The distribution of general characteristics, blood Hg levels, and liver enzyme levels was assessed by descriptive statistics such as frequency, proportion, arithmetic/geometric mean and standard deviation (SD), minimum, 25th percentile, median, 75th percentile, and maximum. Because the distributions of blood Hg and liver enzyme levels were right-skewed, log-transformed values were used to test the differences according to the subjects’ characteristics, and the Student t-test and analysis of variance were used.
- A generalized linear model (GLM) was used to analyze cross-sectional associations between blood Hg and liver enzyme levels at baseline and follow-up. Nonlinear relationships were examined using a generalized additive mixed model (GAMM). In this model, the base-2 log blood Hg level was given a cubic regression spline function. The population-averaged association over the period of the study between blood Hg and liver enzyme levels was analyzed using a generalized estimating equations (GEE) model considering repeated measures for the same individuals stratified by gender. In the GEE analysis, the interaction of blood Hg and the frequency of alcohol consumption in association with liver enzymes was evaluated.
- In all analyses, natural log-transformed liver enzyme levels and base 2 log-transformed blood Hg levels were used as response and predictor variables, respectively. Survey wave, gender, age, income, fish consumption, smoking, frequency of alcohol consumption, and past medical history were considered as covariates in the regression models, and individuals were included in GAMM as random intercepts. Each variable corresponded to values measured at each survey wave in the GLM, and were considered to be time-varying covariates in the GAMM and GEE. The significance level for tests was 0.05, and R version 3.3.3 (Comprehensive R Archive Network: http://cran.r-project.org) was used.
METHODS
- In the baseline survey, the geometric mean (geometric SD) levels of blood Hg, ALT, AST, and GGT in all participants were 4.33 μg/L (1.94 μg/L), 21.2 IU/L (1.4 IU/L), 18.3 IU/L (1.7 IU/L), and 23.4 IU/L (2.0 IU/L), respectively. Blood Hg levels were significantly higher in men than women, as age increased, in smokers than non-smokers, as fish intake increased, as the frequency of alcohol consumption increased, and in individuals with a history of HTN or TB. All 3 liver enzymes were significantly higher in men than women, as age increased, in smokers than non-smokers, as the frequency of alcohol consumption increased, and in those with a history of HTN. ALT and GGT were significantly higher in those with a history of DM, ALT was significantly higher in those with a history of TB, and AST and ALT were significantly higher in those with a history of hepatitis B than their respective counterparts (Table 1).
- Compared to the baseline values, the follow-up concentrations of blood Hg and AST decreased, that of ALT increased, and that of GGT was similar (p<0.001, p<0.001, p<0.001, and p=0.38, respectively) (Table 2). Descriptive findings regarding the distribution of the change in levels of each enzyme, as well as Hg concentrations, from the baseline to follow-up are shown in Supplemental Table 1.
- Significant positive associations were found between blood Hg and GGT at baseline and follow-up (in all participants and in women), but no significant associations with AST or ALT were observed. Although the association of blood Hg with AST and ALT in all participants showed a significant difference between the 2 survey waves, there was no significant difference after stratification by gender (Table 3). There was no statistically significant association between blood Hg levels at baseline or changes in blood Hg and changes in liver enzyme levels between surveys (Supplemental Table 2).
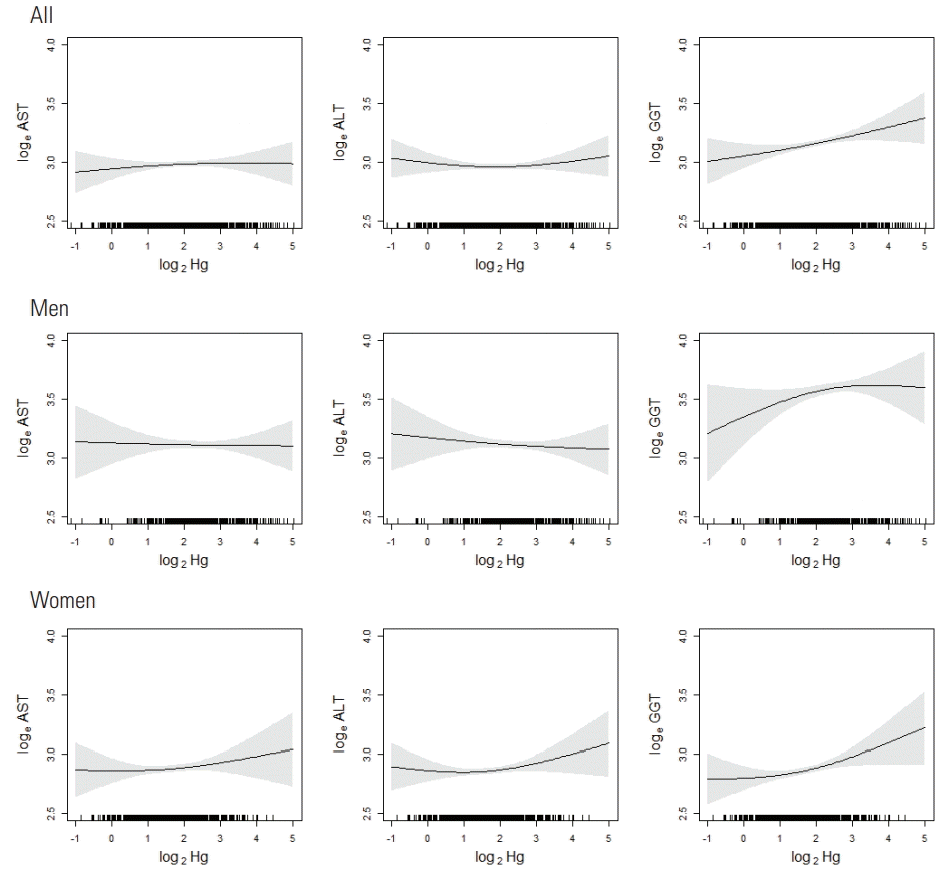
- The relationships between blood Hg levels and liver enzyme levels are illustrated in Figure 1. In men, AST and ALT showed little change as blood Hg levels increased, and GGT showed an increase with a downward curvature. In women, AST, ALT, and GGT showed an increase with an upward curvature.
- The associations between blood Hg and AST or ALT, when adjusted for other covariates and individual correlations in repeated measurements, were not significant in either gender (Table 4). GGT showed a significant association with blood Hg levels in women and borderline significance in men. The association between blood Hg and AST was significantly different by gender (p for interaction=0.02).
- In women who consumed alcohol ≥2-3 times per week, all 3 liver enzymes showed significant positive associations with blood Hg levels, and the effects of blood Hg and frequency of alcohol consumption on GGT showed a significant synergistic interaction (p for interaction=0.008) (Table 4). The frequency and amount of alcohol consumption were strongly correlated (Supplemental Table 3), and the results were similar to those of drinking frequency when the analysis was repeated using the amount of alcohol consumed (Supplemental Table 4).
RESULTS
- In this study, higher blood Hg levels were associated with higher serum GGT levels in men and women. Especially in women who drank alcohol frequently, higher blood Hg levels were significantly associated with elevated AST and ALT levels, as well as GGT, and a significant synergistic effect between Hg and alcohol consumption on liver enzyme levels was observed.
- The geometric mean values of the blood Hg at baseline and follow-up were 4.33 μg/L and 3.18 μg/L, respectively. In the 2012 and 2015 KoNEHS data, the geometric mean levels of blood Hg were reported as 3.08 μg/L and 3.11 μg/L, respectively, and Seo et al. [5] and Lee et al. [12] reported values of 3.08 μg/L and 3.99 μg/L, respectively. Compared to those previous Korean studies, the baseline concentration in the present study was higher and the concentration at follow-up was similar.
- In previous epidemiological studies, blood Hg showed a significant positive association with ALT in NHANES 2003-2004 [11], and with AST and ALT in KNHANES 2008-2012 [12]. A recent study [13] reported significantly higher AST levels in the highest quarter of blood Hg levels than in the lowest quarter, and the odds of high ALT levels (>35 IU/L) were 3.10 times higher in the highest quarter of blood Hg levels than in the lowest quarter in a repeated-measures study of Koreans over 60 years old. However, other studies have reported no association [9,10], and conflicting results have been reported depending on whether liver dysfunction was defined as a continuous or dichotomous variable within the same study [13]. Significantly higher GGT levels were found in the second-highest and highest quarters of blood Hg levels than in the lowest quarter [13], and the odds of high GGT (>47 IU/L for men and >21 IU/L for women) were 2.59 times higher for men and 2.03 times higher for women in the highest quarter of blood Hg levels than in the lowest quarter [14]. The differences in results among studies may be explained by various factors, such as differences in the adjusted confounding variables, study design, sample size, and whether effect modifiers such as alcohol consumption and gender were considered. In the present study, an interaction between blood Hg levels and alcohol consumption was observed for GGT in women, which was consistent with previous results. This may have been because GGT is a more sensitive biomarker for alcohol consumption than AST or ALT [13].
- The biological mechanism of associations between Hg exposure and liver dysfunction is mainly explained by oxidative stress, cell death, and impairment of metabolism. In a study using NHANES 1988-1994 data, negative associations of GGT with antioxidants including a-carotene, h-carotene, h-cryptoxanthin, zeaxanthin/lutein, lycopene, and vitamin C were reported, suggesting that GGT may be considered an early oxidative stress marker [16]. In an animal study of male rats exposed to Hg, AST, ALT, and GGT were significantly elevated, and marked damage or necrotic changes were observed in most of the liver tissue samples upon a histological examination [17]. Degenerative changes and large lysed areas in liver parenchyma in Hg-exposed zebrafish have been observed [18]. In addition, Hg exposure in zebrafish induced deregulation of oxidative stress, intrinsic apoptotic pathways, and nuclear receptor and kinase activity [19], suggesting that gluconeogenesis and adipogenesis occur in these processes and that hepatotoxicity occurs through cell death, mitochondrial dysfunction, endocrine disruption, and metabolic disorders. Koenig and Seneff [20] proposed that GGT, an indicator of alcohol-related liver disease, is an important biomarker of cellular antioxidant inadequacy. Therefore, the results of the present study that GGT was susceptible to elevated blood Hg levels, mediated through an interaction with alcohol consumption, imply that Hg may induce hepatotoxicity through oxidative stress.
- The present study has several strengths. First, we considered fish consumption as a potential confounder when examining associations between blood Hg levels and liver function. Fish is a major source of Hg, and 80-90% of the organic Hg in the human body comes from fish and shellfish intake [21]. Because methylmercury is highly lipophilic and bioaccumulative, methylmercury exposure occurs mostly via food intake, such as fish consumption. Fish consumption increases total blood Hg, while the antioxidant effects of fish oil and nutrients may offset the harmful effect of Hg on liver enzymes. Most previous studies have not considered fish consumption [13,14]. Second, we examined the possibility of a nonlinear association between Hg levels and liver enzyme levels. Most previous epidemiological studies have examined linear relationships or Hg levels divided into quantiles, which could lead to biased results if these variables have a nonlinear relationship. In the present study, we did not find evidence of a nonlinear association between Hg levels and liver function, although further studies are warranted to replicate our findings. Third, we analyzed the data with consideration of the repeatedly measured and longitudinal structure of the data, whereas most previous studies on the associations between Hg and liver enzymes were purely cross-sectional.
- Nonetheless, this study has some limitations. First, we did not have information on organic Hg per se; instead, we measured the total Hg concentration. However, it is known that organic Hg accounts for most of the Hg content in the human body [21]. Specifically, methylmercury is known to contribute to about 90% of the total blood Hg concentration when the total Hg level is about 4 μg/L or more, and the total Hg in the general population is known to reflect organic Hg exposure unless individuals are exposed to high concentrations of inorganic Hg [22]. Therefore, in the present study, the analysis of total blood Hg could also be interpreted meaningfully as an analysis of organic Hg to some extent. Second, fish consumption was measured by the 24-hour diet recall method. This method was based on 2 non-consecutive days of diet recall, and the average intake (g/kg/d) was calculated; this method may have introduced uncertainty in measurements of fish intake. Third, the frequency of alcohol consumption for the past year was measured by the questionnaire, whereas information on alcohol consumption the day before the test was not obtained, although this could have affected liver enzyme levels. However, because the introductory brochure provided to the enrollees contained information on drinking restrictions before the test, this impact should have been minimal.
- In conclusion, total blood Hg exposure was associated with an increase in liver enzymes, particularly in women heavy drinkers. Women may be more sensitive to the hepatotoxic effects of Hg, and Hg exposure showed a synergistic effect on liver damage with a high frequency of alcohol consumption.
DISCUSSION
ACKNOWLEDGEMENTS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
Notes
SUPPLEMENTAL MATERIAL
Supplemental Table 1.
Supplemental Table 2.
Supplemental Table 3.
Supplemental Table 4.
| n (%) | Hg (μg/L) | p-value1 | AST (IU/L) | p-value1 | ALT (IU/L) | p-value1 | GGT (IU/L) | p-value1 | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 508 (100.0) | 4.33 (1.94) | 21.2 (1.4) | 18.3 (1.7) | 23.4 (2.0) | ||||
| Gender | |||||||||
| Men | 211 (41.5) | 5.71 (1.94) | 23.2 (1.4) | 22.6 (1.6) | 36.1 (2.0) | ||||
| Women | 297 (58.5) | 3.55 (1.80) | <0.001 | 19.8 (1.4) | <0.001 | 15.7 (1.6) | <0.001 | 17.2 (1.7) | <0.001 |
| Age at baseline (y) | |||||||||
| ≤29 | 53 (10.4) | 2.87 (1.70) | 19.2 (1.5) | 15.1 (2.1) | 17.5 (1.7) | ||||
| 30-39 | 73 (14.4) | 3.99 (1.77) | 18.3 (1.3) | 16.5 (1.6) | 20.4 (1.9) | ||||
| 40-49 | 113 (22.2) | 4.57 (2.06) | 21.3 (1.5) | 19.3 (1.7) | 26.0 (2.2) | ||||
| 50-59 | 135 (26.6) | 4.22 (1.86) | 21.8 (1.4) | 18.9 (1.6) | 22.3 (1.9) | ||||
| ≥60 | 134 (26.4) | 5.20 (1.95) | <0.001 | 23.1 (1.3) | <0.001 | 19.3 (1.5) | 0.008 | 27.1 (2.0) | <0.001 |
| Residence | |||||||||
| Rural area | 305 (60.0) | 4.49 (1.89) | 21.0 (1.4) | 18.5 (1.7) | 24.1 (2.1) | ||||
| Urban area | 203 (40.0) | 4.09 (2.01) | 0.12 | 21.4 (1.4) | 0.57 | 18.1 (1.7) | 0.64 | 22.4 (1.9) | 0.24 |
| Income (104 KRW/mo) | |||||||||
| <100 | 83 (17.1) | 4.34 (2.04) | 22.0 (1.4) | 18.3 (1.5) | 25.5 (2.0) | ||||
| 100-200 | 112 (23.0) | 4.48 (1.96) | 22.2 (1.4) | 19.3 (1.8) | 25.5 (2.2) | ||||
| 200-300 | 140 (28.8) | 4.32 (1.82) | 21.3 (1.5) | 17.9 (1.7) | 22.0 (2.1) | ||||
| 300-400 | 77 (15.8) | 4.42 (1.99) | 20.6 (1.3) | 18.5 (1.6) | 24.4 (1.8) | ||||
| 400-600 | 55 (11.3) | 3.84 (1.94) | 19.1 (1.3) | 17.0 (1.7) | 19.8 (1.8) | ||||
| ≥600 | 19 (3.9) | 6.06 (2.10) | 0.23 | 20.4 (1.5) | 0.14 | 20.7 (1.9) | 0.60 | 24.4 (2.1) | 0.19 |
| History of smoking | |||||||||
| No | 332 (65.9) | 3.74 (1.83) | 20.4 (1.4) | 16.5 (1.6) | 18.6 (1.7) | ||||
| Yes | 172 (34.1) | 5.76 (1.96) | <0.001 | 22.8 (1.5) | <0.001 | 22.3 (1.6) | <0.001 | 36.2 (2.1) | <0.001 |
| Frequency of alcohol consumption | |||||||||
| None | 124 (24.6) | 3.90 (1.95) | 20.9 (1.4) | 17.9 (1.6) | 19.6 (1.7) | ||||
| <1/mo | 74 (14.7) | 3.61 (1.90) | 19.7 (1.4) | 16.1 (1.6) | 17.6 (1.7) | ||||
| 1/mo | 68 (13.5) | 4.41 (1.74) | 20.2 (1.4) | 18.0 (1.7) | 19.9 (1.9) | ||||
| 2-4 times/mo | 117 (23.2) | 3.97 (1.94) | 19.7 (1.3) | 17.1 (1.6) | 21.6 (1.8) | ||||
| 2-3 times/wk | 74 (14.7) | 5.29 (1.86) | 23.5 (1.5) | 21.0 (1.8) | 32.8 (2.0) | ||||
| 4+ times/wk | 48 (9.5) | 6.75 (1.89) | <0.001 | 26.2 (1.5) | <0.001 | 23.0 (1.6) | <0.001 | 51.9 (2.5) | <0.001 |
| Fish consumption (g/kg/d) | |||||||||
| 1st quarter (<0.20) | 117 (23.1) | 3.93 (1.91) | 22.0 (1.4) | 19.7 (1.7) | 27.9 (2.1) | ||||
| 2nd quarter (0.20-0.72) | 139 (27.4) | 3.90 (1.95) | 20.9 (1.5) | 17.5 (1.7) | 22.4 (2.0) | ||||
| 3rd quarter (0.73-1.45) | 124 (24.5) | 4.14 (1.95) | 20.8 (1.4) | 17.5 (1.7) | 20.7 (1.9) | ||||
| 4th quarter (≥1.46) | 127 (25.0) | 5.55 (1.83) | <0.001 | 21.1 (1.3) | 0.61 | 18.9 (1.6) | 0.18 | 23.8 (2.0) | 0.007 |
| Past medical history | |||||||||
| Hypertension | |||||||||
| No | 362 (73.6) | 4.07 (1.91) | 20.5 (1.4) | 17.4 (1.7) | 21.3 (1.9) | ||||
| Yes | 130 (26.4) | 5.13 (2.00) | 0.001 | 22.7 (1.4) | 0.003 | 20.7 (1.6) | 0.001 | 29.4 (2.1) | <0.001 |
| Diabetes mellitus | |||||||||
| No | 433 (88.5) | 4.34 (1.97) | 20.8 (1.4) | 17.8 (1.7) | 22.6 (2.0) | ||||
| Yes | 56 (11.5) | 4.27 (1.82) | 0.86 | 22.6 (1.4) | 0.08 | 21.7 (1.7) | 0.007 | 29.0 (2.0) | 0.01 |
| Kidney disease | |||||||||
| No | 455 (94.6) | 4.36 (1.97) | 21.0 (1.4) | 18.4 (1.7) | 23.5 (2.0) | ||||
| Yes | 26 (5.4) | 4.33 (1.80) | 0.96 | 20.5 (1.2) | 0.70 | 15.7 (1.3) | 0.12 | 19.4 (1.7) | 0.18 |
| Tuberculosis | |||||||||
| No | 453 (94.4) | 4.26 (1.95) | 20.9 (1.4) | 17.9 (1.7) | 23.0 (2.0) | ||||
| Yes | 27 (5.6) | 6.15 (1.94) | 0.006 | 23.4 (1.4) | 0.08 | 24.1 (1.7) | 0.003 | 28.6 (2.0) | 0.11 |
| Dyslipidemia | |||||||||
| No | 448 (92.8) | 4.34 (1.96) | 21.0 (1.4) | 18.2 (1.7) | 23.2 (2.0) | ||||
| Yes | 35 (7.2) | 4.35 (1.82) | 0.99 | 21.0 (1.3) | 0.95 | 19.3 (1.7) | 0.53 | 24.3 (1.9) | 0.72 |
| Hepatitis B | |||||||||
| No | 439 (92.0) | 4.38 (1.93) | 20.8 (1.4) | 18.0 (1.7) | 23.2 (2.0) | ||||
| Yes | 38 (8.0) | 4.44 (2.07) | 0.91 | 23.1 (1.4) | 0.06 | 21.6 (1.6) | 0.03 | 22.7 (1.8) | 0.85 |
Values are presented as geometric mean (geometric standard deviation).
Hg, mercury; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyltransferase; KRW, Korean won.
1 p-values were calculated using the t-test or analysis of variance with natural log-transformed values of Hg, AST, ALT, and GGT.
| Survey wave (periods) | Blood Hg or liver enzymes | Mean (SD) | gMean (gSD) | Min | 1st Q | Median | 3rd Q | Max | p-value1 |
|---|---|---|---|---|---|---|---|---|---|
| Baseline (2010-2011) | Hg (μg/L) | 5.44 (4.40) | 4.33 (1.94) | 0.46 | 2.90 | 4.22 | 6.54 | 41.95 | |
| AST (IU/L) | 22.8 (12.9) | 21.2 (1.4) | 10.0 | 17.0 | 20.0 | 24.0 | 187.0 | ||
| ALT (IU/L) | 21.3 (15.2) | 18.3 (1.7) | 5.0 | 13.0 | 17.0 | 24.0 | 153.0 | ||
| GGT (IU/L) | 31.9 (37.8) | 23.4 (2.0) | 7.0 | 14.0 | 20.0 | 36.0 | 473.0 | ||
| Follow-up (2014-2015) | Hg (μg/L) | 4.08 (3.44) | 3.18 (2.00) | 0.31 | 2.02 | 3.05 | 5.01 | 26.90 | <0.001 |
| AST (IU/L) | 21.8 (16.2) | 18.3 (1.7) | 2.5 | 13.0 | 17.0 | 25.0 | 169.0 | <0.001 | |
| ALT (IU/L) | 22.5 (10.8) | 20.9 (1.4) | 9.0 | 16.0 | 20.0 | 25.0 | 93.0 | <0.001 | |
| GGT (IU/L) | 32.7 (42.2) | 23.8 (2.0) | 6.0 | 14.0 | 21.0 | 36.0 | 589.0 | 0.38 |
Hg, mercury; SD, standard deviation; gMean, geometric mean; gSD, geometric standard deviation; Min, minimum; 1st Q, 1st quartile; 3rd Q, 3rd quartile; Max, maximum; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyltransferase.
1 p-values were calculated using the paired t-test for differences in the geometric mean between survey waves.
| Survey wave |
AST |
ALT |
GGT |
||||
|---|---|---|---|---|---|---|---|
| % change (95% CI)2 | p for interaction3 | % change (95% CI)2 | p for interaction3 | % change (95% CI)2 | p for interaction3 | ||
| All | Baseline | -1.2 (-4.7, 2.5) | 2.2 (-3.3, 7.9) | 8.3 (1.8, 15.2)* | |||
| Follow-up | 3.6 (-2.2, 9.8) | 0.03 | -0.6 (-4.2, 3.1) | 0.003 | 10.3 (3.5, 17.6)* | 0.60 | |
| Men | Baseline | -2.5 (-7.9, 3.3) | 0.7 (-7.3, 9.3) | 6.1 (-4.8, 18.2) | |||
| Follow-up | 0.9 (-7.0, 9.4) | 0.69 | -3.1 (-8.6, 2.8) | 0.16 | 9.4 (-2.0, 22.2) | 0.70 | |
| Womene | Baseline | -0.5 (-5.3, 4.6) | 3.0 (-4.4, 11.0) | 9.7 (1.7, 18.2)* | |||
| Follow-up | 5.6 (-2.8, 14.7) | 0.12 | 2.1 (-2.9, 7.3) | 0.54 | 12.3 (3.5, 21.8)* | 0.61 | |
Hg, mercury; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyltransferase.
1 Generalized linear model adjusted for gender (in all), age, income, fish consumption, smoking, frequency of alcohol consumption, and past medical history of hypertension, diabetes mellitus, tuberculosis, or hepatitis B measured at each period.
2 % change of liver enzyme per doubling of blood Hg levels.
3 Interactions between blood Hg levels and survey wave in association with liver function indices were evaluated by the interaction term in the generalized estimating equation model.
* p<0.05.
|
AST |
ALT |
GGT |
|||||
|---|---|---|---|---|---|---|---|
| % change (95% CI)1 | p for interaction2 | % change (95% CI)1 | p for interaction2 | % change (95% CI)1 | p for interaction2 | ||
| Men | Crude | 1.8 (-2.4, 6.1) | 0.6 (-3.8, 5.3) | 12.7 (3.2, 23.0)* | |||
| Model 1 | 1.6 (-2.7, 6.0) | -0.1 (-4.9, 4.8) | 10.4 (-0.1, 22.1)† | ||||
| Model 2 | 1.3 (-3.3, 6.0) | -0.8 (-5.5, 4.1) | 7.5 (-1.8, 17.7) | ||||
| Model 3 | -0.1 (-4.7, 4.7) | -1.2 (-5.6, 3.4) | 8.1 (-0.5, 17.4)† | ||||
| Alcohol consumption frequency3 | |||||||
| ≤2-4 times/mo | 3.6 (-2.3, 9.8) | 2.9 (-2.5, 8.6) | 7.6 (-2.0, 18.2) | ||||
| ≥2-3 times/wk | -1.7 (-9.0, 6.2) | 0.88 | -2.7 (-10.5, 5.7) | 0.31 | 13.5 (-2.0, 31.4)† | 0.39 | |
| Women | Crude | 6.9 (2.8, 11.2)* | 0.9 (-2.9, 4.8) | 7.0 (1.1, 13.3)* | |||
| Model 1 | 2.1 (-1.9, 6.2) | 1.8 (-2.2, 6.0) | 8.1 (1.4, 15.2)* | ||||
| Model 2 | 2.2 (-1.7, 6.2) | 1.5 (-2.4, 5.6) | 9.1 (2.3, 16.3)* | ||||
| Model 3 | 3.2 (-1.1, 7.6) | 2.4 (-1.7, 6.6) | 11.0 (4.5, 18.0)* | ||||
| Alcohol consumption frequency3 | |||||||
| ≤2-4 times/mo | 2.5 (-2.2, 7.4) | 2.0 (-2.4, 6.7) | 7.8 (1.2, 14.7)* | ||||
| ≥2-3 times/wk | 10.6 (4.2, 17.5)* | 0.22 | 7.7 (1.1, 14.7)* | 0.37 | 37.5 (15.2, 64.3)* | 0.008 | |
| p for interaction between genders4 | 0.02 | 0.53 | 0.82 | ||||
Model 1 included age, income, and study period in the crude model; Model 2 included fish consumption, smoking, and frequency of alcohol consumption in addition to model 1; Model 3 included past medical history of hypertension, diabetes mellitus, tuberculosis, or hepatitis B in addition to model 2. In each model, age, income, period, fish consumption, smoking, and frequency of alcohol consumption, as well as blood Hg and liver enzyme levels, were considered to be time-varying covariates.
Hg, mercury; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyltransferase.
1 % change of liver enzyme per doubling of blood Hg level.
2 The multiplicative interaction between blood Hg levels and frequency of alcohol consumption in association with liver enzymes was evaluated by the interaction term in the corresponding model.
3 After stratification by frequency of alcohol consumption, the same covariates as model 3 were included.
4 The multiplicative interaction between blood Hg levels and gender in association with liver enzymes was evaluated by the interaction term in the corresponding model (model 3).
† p<0.1,
* p<0.05.
- 1. Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A. Mercury exposure and heart diseases. Int J Environ Res Public Health 2017;14(1):E74Article
- 2. World Health Organization. Exposure to mercury: a major public health concern, preventing disease through healthy environment. Geneva: World Health Organization; 2007. p. 1
- 3. Yaginuma-Sakurai K, Murata K, Iwai-Shimada M, Nakai K, Kurokawa N, Tatsuta N, et al. Hair-to-blood ratio and biological half-life of mercury: experimental study of methylmercury exposure through fish consumption in humans. J Toxicol Sci 2012;37(1):123-130ArticlePubMed
- 4. Awata H, Linder S, Mitchell LE, Delclos GL. Biomarker levels of toxic metals among Asian populations in the United States: NHANES 2011-2012. Environ Health Perspect 2017;125(3):306-313ArticlePubMed
- 5. Seo JW, Kim BG, Kim YM, Kim RB, Chung JY, Lee KM, et al. Trend of blood lead, mercury, and cadmium levels in Korean population: data analysis of the Korea National Health and Nutrition Examination Survey. Environ Monit Assess 2015;187(3):146ArticlePubMed
- 6. Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 1995;25(1):1-24ArticlePubMed
- 7. Mahaffey KR. Mercury exposure: medical and public health issues. Trans Am Clin Climatol Assoc 2005;116: 127-154PubMedPMC
- 8. Fernandes Azevedo B, Barros Furieri L, Peçanha FM, Wiggers GA, Frizera Vassallo P, Ronacher Simões M, et al. Toxic effects of mercury on the cardiovascular and central nervous systems. J Biomed Biotechnol 2012;2012: 949048ArticlePubMedPMCPDF
- 9. Futatsuka M, Kitano T, Nagano M, Inaoka T, Arimatsu Y, Ueno T, et al. An epidemiological study with risk analysis of liver diseases in the general population living in a methyl mercury polluted area. J Epidemiol Community Health 1992;46(3):237-240ArticlePubMedPMC
- 10. Poursafa P, Ataee E, Motlagh ME, Ardalan G, Tajadini MH, Yazdi M, et al. Association of serum lead and mercury level with cardiometabolic risk factors and liver enzymes in a nationally representative sample of adolescents: the CASPIAN-III study. Environ Sci Pollut Res Int 2014;21(23):13496-13502ArticlePubMed
- 11. Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ Health Perspect 2010;118(12):1735-1742ArticlePubMedPMC
- 12. Lee H, Kim Y, Sim CS, Ham JO, Kim NS, Lee BK. Associations between blood mercury levels and subclinical changes in liver enzymes among South Korean general adults: analysis of 2008-2012 Korean National Health and Nutrition Examination Survey data. Environ Res 2014;130: 14-19ArticlePubMed
- 13. Lee MR, Lim YH, Lee BE, Hong YC. Blood mercury concentrations are associated with decline in liver function in an elderly population: a panel study. Environ Health 2017;16(1):17ArticlePubMedPMCPDF
- 14. Seo MS, Lee HR, Shim JY, Kang HT, Lee YJ. Relationship between blood mercury concentrations and serum γ-glutamyltrans-peptidase level in Korean adults using data from the 2010 Korean National Health and Nutrition Examination Survey. Clin Chim Acta 2014;430: 160-163ArticlePubMed
- 15. Lim JA, Kwon HJ, Ha M, Kim H, Oh SY, Kim JS, et al. Korean research project on the integrated exposure assessment of hazardous substances for food safety. Environ Health Toxicol 2015;30: e2015004ArticlePubMedPMCPDF
- 16. Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med 2004;37(7):1018-1023ArticlePubMed
- 17. Wadaan MA. Effects of mercury exposure on blood chemistry and liver histopathology of male rats. J Pharmacol Toxicol 2009;4(3):126-131Article
- 18. Macirella R, Guardia A, Pellegrino D, Bernabò I, Tronci V, Ebbesson LO, et al. Effects of two sublethal concentrations of mercury chloride on the morphology and metallothionein activity in the liver of zebrafish (Danio rerio). Int J Mol Sci 2016;17(3):361ArticlePubMedPMC
- 19. Ung CY, Lam SH, Hlaing MM, Winata CL, Korzh S, Mathavan S, et al. Mercury-induced hepatotoxicity in zebrafish: in vivo mechanistic insights from transcriptome analysis, phenotype anchoring and targeted gene expression validation. BMC Genomics 2010;11: 212ArticlePubMedPMC
- 20. Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers 2015;2015: 818570ArticlePubMedPMCPDF
- 21. Hong YS, Kim YM, Lee KE. Methylmercury exposure and health effects. J Prev Med Public Health 2012;45(6):353-363ArticlePubMedPMCPDF
- 22. Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect 2004;112(5):562-570ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Selenium and zinc alleviate hepatotoxicity induced by heavy metal mixture (cadmium, mercury, lead and arsenic) via attenuation of inflammo‐oxidant pathways
Harrison Ozoani, Anthonet N. Ezejiofor, Kenneth O. Okolo, Chinna N. Orish, Ana Cirovic, Aleksandar Cirovic, Orish E. Orisakwe
Environmental Toxicology.2024; 39(1): 156. CrossRef - Deleterious effects of mercury contamination on immunocompetence, liver function and egg volume in an antarctic seabird
Andrés E. Ibañez, William F. Mills, Paco Bustamante, Lara M. Morales, Diego S. Torres, Beatriz D' Astek, Rocío Mariano-Jelicich, Richard A. Phillips, Diego Montalti
Chemosphere.2024; 346: 140630. CrossRef - Letter to the editor: In utero exposure to mercury is associated with increased susceptibility to liver injury and inflammation in childhood
Muhammad Zawar Asif, Muhammad Umair, Muhammad Shehryar
Hepatology.2023; 77(2): E38. CrossRef - Associations of blood metals with liver function: Analysis of NHANES from 2011 to 2018
Wenjie Li, Xinyan Li, Jing Su, Han Chen, Ping Zhao, Haisheng Qian, Xin Gao, Qiang Ye, Guoxin Zhang, Xuan Li
Chemosphere.2023; 317: 137854. CrossRef - Association of Blood Mercury Level with Liver Enzymes in Korean Adults: An Analysis of 2015–2017 Korean National Environmental Health Survey
Jin-Wook Chung, Dilaram Acharya, Jitendra Kumar Singh, Joon Sakong
International Journal of Environmental Research and Public Health.2023; 20(4): 3290. CrossRef - Environment-wide association study of elevated liver enzymes: results from the Korean National Environmental Health Survey 2018–2022
Youngchan Chi, Jong-Tae Park, Sewhan Na, Kyeongmin Kwak
Annals of Occupational and Environmental Medicine.2023;[Epub] CrossRef - Dissecting the role of cadmium, lead, arsenic, and mercury in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
Alexey A. Tinkov, Michael Aschner, Abel Santamaria, Alfred R. Bogdanov, Yousef Tizabi, Miriam B. Virgolini, Ji-Chang Zhou, Anatoly V. Skalny
Environmental Research.2023; 238: 117134. CrossRef - Lead, mercury, and cadmium exposures are associated with obesity but not with diabetes mellitus: Korean National Environmental Health Survey (KoNEHS) 2015–2017
Min Kyong Moon, Inae Lee, Aram Lee, Hyunwoong Park, Min Joo Kim, Sunmi Kim, Yoon Hee Cho, Sooyeon Hong, Jiyoung Yoo, Gi Jeong Cheon, Kyungho Choi, Young Joo Park, Jeongim Park
Environmental Research.2022; 204: 111888. CrossRef - Identification source and human health risk assessment of potentially toxic metal in soil samples around karst watershed of Pangkajene, Indonesia
Anwar Mallongi, Ratna Dwi Puji Astuti, Ridwan Amiruddin, Muhammad Hatta, Annisa Utami Rauf
Environmental Nanotechnology, Monitoring & Management.2022; 17: 100634. CrossRef - Effects of lead and cadmium co-exposure on liver function in residents near a mining and smelting area in northwestern China
Jun Yan, Honglong Zhang, Jingping Niu, Bin Luo, Haiping Wang, Meng Tian, Xun Li
Environmental Geochemistry and Health.2022; 44(11): 4173. CrossRef - A novel nano-palladium embedded on the mesoporous silica nanoparticles for mercury vapor removal from air by the gas field separation consolidation process
Hamid Shirkhanloo, Farideh Golbabaei, Amir Vahid, Ali Faghihi Zarandi
Applied Nanoscience.2022; 12(5): 1667. CrossRef - Associations between lead, cadmium, mercury, and arsenic exposure and alanine aminotransferase elevation in the general adult population: an exposure–response analysis
Xiaoming Zhou, Yijun Feng, Zonglin Gong
Environmental Science and Pollution Research.2022; 29(35): 53633. CrossRef - Cadmium, lead, and mercury mixtures interact with non-alcoholic fatty liver diseases
Hai Duc Nguyen, Min-Sun Kim
Environmental Pollution.2022; 309: 119780. CrossRef - Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic
Mahdi Balali-Mood, Kobra Naseri, Zoya Tahergorabi, Mohammad Reza Khazdair, Mahmood Sadeghi
Frontiers in Pharmacology.2021;[Epub] CrossRef - Association between Blood Mercury Levels and Non-Alcoholic Fatty Liver Disease in Non-Obese Populations: The Korean National Environmental Health Survey (KoNEHS) 2012–2014
Yun-Jung Yang, Eun-Jung Yang, Kyongjin Park, Subin Oh, Taehyen Kim, Yeon-Pyo Hong
International Journal of Environmental Research and Public Health.2021; 18(12): 6412. CrossRef - In Utero Exposure to Mercury Is Associated With Increased Susceptibility to Liver Injury and Inflammation in Childhood
Nikos Stratakis, Lucy Golden‐Mason, Katerina Margetaki, Yinqi Zhao, Damaskini Valvi, Erika Garcia, Léa Maitre, Sandra Andrusaityte, Xavier Basagana, Eva Borràs, Mariona Bustamante, Maribel Casas, Serena Fossati, Regina Grazuleviciene, Line Småstuen Haug,
Hepatology.2021; 74(3): 1546. CrossRef - The Relationship Between Embryotoxicity and Oxidative Stress Produced by Aluminum, Iron, Mercury, and Their Mixture on Cyprinus carpio
Selene Cano-Viveros, Marcela Galar-Martínez, Eloy Gasca-Pérez, Sandra García-Medina, Karina Ruiz-Lara, Leobardo Manuel Gómez-Oliván, Hariz Islas-Flores
Water, Air, & Soil Pollution.2021;[Epub] CrossRef - Tissue distribution of mercury and copper after Aarogyavardhini Vati treatment in rat model of CCl4 induced chronic hepatotoxicity
Shrirang Jamadagni, Pallavi Jamadagni, Binita Angom, Dhirendranath Mondal, Sachchidanand Upadhyay, Sudesh Gaidhani, Jayram Hazra
Journal of Ayurveda and Integrative Medicine.2020; 11(4): 508. CrossRef - Mercury exposure and premature mortality in the Grassy Narrows First Nation community: a retrospective longitudinal study
Aline Philibert, Myriam Fillion, Donna Mergler
The Lancet Planetary Health.2020; 4(4): e141. CrossRef - The sex-specific effects of blood lead, mercury, and cadmium levels on hepatic steatosis and fibrosis: Korean nationwide cross-sectional study
Seung Min Chung, Jun Sung Moon, Ji Sung Yoon, Kyu Chang Won, Hyoung Woo Lee
Journal of Trace Elements in Medicine and Biology.2020; 62: 126601. CrossRef - Blood mercury and liver enzymes: A pan-India retrospective correlation study
Krishnakumar Sivapandi, Amruta Velumani, Kallathikumar Kallathiyan, Sandhya Iyer, Prachi Sinkar
Toxicology and Industrial Health.2020; 36(12): 1019. CrossRef - Mercury in cetaceans: Exposure, bioaccumulation and toxicity
Joanna L. Kershaw, Ailsa J. Hall
Science of The Total Environment.2019; 694: 133683. CrossRef - Associations between mercury exposure and the risk of nonalcoholic fatty liver disease (NAFLD) in US adolescents
Runsen Chen, Yang Xu, Cheng Xu, Yaqin Shu, Siyu Ma, Changgui Lu, Xuming Mo
Environmental Science and Pollution Research.2019; 26(30): 31384. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


