Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 49(6); 2016 > Article
-
Review
Breast Density and Risk of Breast Cancer in Asian Women: A Meta-analysis of Observational Studies -
Jong-Myon Bae
 , Eun Hee Kim
, Eun Hee Kim
-
Journal of Preventive Medicine and Public Health 2016;49(6):367-375.
DOI: https://doi.org/10.3961/jpmph.16.054
Published online: October 21, 2016
Department of Preventive Medicine, Jeju National University School of Medicine, Jeju, Korea
- Corresponding author: Jong-Myon Bae, MD, PhD 102 Jejudaehak-ro, Jeju 63241, Korea Tel: +82-64-755-5567,Fax: +82-64-702-2687 E-mail: jmbae@jejunu.ac.kr
Copyright © 2016 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- The established theory that breast density is an independent predictor of breast cancer risk is based on studies targeting white women in the West. More Asian women than Western women have dense breasts, but the incidence of breast cancer is lower among Asian women. This meta-analysis investigated the association between breast density in mammography and breast cancer risk in Asian women.
-
Methods
- PubMed and Scopus were searched, and the final date of publication was set as December 31, 2015. The effect size in each article was calculated using the interval-collapse method. Summary effect sizes (sESs) and 95% confidence intervals (CIs) were calculated by conducting a meta-analysis applying a random effect model. To investigate the dose-response relationship, random effect dose-response meta-regression (RE-DRMR) was conducted.
-
Results
- Six analytical epidemiology studies in total were selected, including one cohort study and five case-control studies. A total of 17 datasets were constructed by type of breast density index and menopausal status. In analyzing the subgroups of premenopausal vs. postmenopausal women, the percent density (PD) index was confirmed to be associated with a significantly elevated risk for breast cancer (sES, 2.21; 95% CI, 1.52 to 3.21; I2=50.0%). The RE-DRMR results showed that the risk of breast cancer increased 1.73 times for each 25% increase in PD in postmenopausal women (95% CI, 1.20 to 2.47).
-
Conclusions
- In Asian women, breast cancer risk increased with breast density measured using the PD index, regardless of menopausal status. We propose the further development of a breast cancer risk prediction model based on the application of PD in Asian women.
- Breast cancer, which has the highest incidence and mortality rate of women cancers globally, imposes a significant disease burden in developing countries [1]. Among breast cancer risk factors, dense breasts found on mammography due to breast epithelium and stroma are known to be a potent risk factor, raising breast cancer risk by four to six times [2-4] according to previous systematic reviews (SRs) [5-9]. However, these SRs have mostly been performed on studies of white women in the West [8,10].
- Asian women, whose breast cancer incidence rate is lower than that of Western white women, have been reported to have dense breasts on mammography more frequently [11-14]. That is, Asian women have denser breasts on mammography, but lower breast cancer incidence than white women [15]. These facts make us doubt the proposal that breast density may be a risk factor in Asian women [16,17]. In particular, the incidence curves of breast cancer in accordance with age are significantly different in Asian women, including Koreans, than in Western women [18-20]. Thus, we can infer that the risk factor of breast density works differently in Asian women than in Western women [17].
- Some studies have reported that breast density on mammography was a risk factor for breast cancer in Asian women [21-23]. However, the risks varied depending on the density measurement index, statistical significance varied for each density interval, and a dose-response relationship has not been shown. As of the end of December 2015, no SR was found that evaluated the association between breast density on mammography and breast cancer risk in Asian women. Thus, the aim of this study was to conduct such an SR.
INTRODUCTION
- Search and Selection of Related Articles
- The final selection criteria for the meta-analysis included analytical epidemiology studies that evaluated the association between breast density levels determined using mammography and breast cancer risk in Asian women. Article selection was conducted in accordance with the preferred reporting items proposed for SRs and meta-analyses, including three stages: searching, screening using titles and abstracts, and evaluating articles [24].
- The databases searched in the first stage were PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and Scopus (www.scopus.com) [25], and the following search formula was applied: [(breast) OR (mammary)] AND [(cancer) OR (neoplasm)] AND [(density) OR (index)] AND [(Asia) OR (women)]. The final date of publication was set at December 31, 2015. Furthermore, a list was made while performing a hand search to investigate whether an SR had already been published evaluating the same hypothesis. The lists from the three search sources were combined to remove duplicates.
- For the 2-stage screening process, the following exclusion criteria were applied based on the titles and abstracts to the summarized list: (1) studies not related to breast cancer, (2) laboratory studies, (3) expert or systematic reviews, and (4) descriptive epidemiological studies including cross-sectional prevalence studies. After the 2-stage screening process and exclusion, the third-stage evaluation was conducted for the remaining articles. For this purpose, the content was evaluated using the full text of each article, and articles that fell into the following categories were excluded sequentially: (5) analytical epidemiology studies that did not provide the information necessary for a meta-analysis, and (6) duplicate studies. Judgment of duplicates was made when the study subjects were selected from the same institution during the same recruiting period. Among duplicates, the article with the largest sample size was selected. The remaining analytical epidemiology studies were ultimately selected for the meta-analysis.
- Statistical Analysis
- The following information was extracted from each article: the nationality of the subjects, the recruiting institution, data sources for cohort construction, menopausal status, number of cancer cases and controls, type of breast density measurement index, and adjusted odds ratios (aORs) or relative risks and their 95% confidence intervals (CIs) for potential confounders at each density level. The measurement indices included the Wolfe classification (Wolfe), percent density (PD, %), volumetric density grade (%), density area (cm2), total breast area (TBA, cm2), absolute dense area (cm2), and mean dense area (MDA, cm2). An article showing aORs divided according to menopausal status and several measurement indices was considered to provide independent datasets for each stratum.
- The effect size (ES) of each dataset to be used in the meta-analysis was calculated using the interval collapsing method (ICM) rather than highest vs. lowest intake method (HLM), because the ICM increases the statistical precision more than the HLM [26]. ICM application adopts the ES and its 95% CI calculated by performing a meta-analysis with a random effect model (REM) on the aOR and its 95% CI presented for each density level within a dataset as the ES for each dataset. After dividing the data into subgroups according to menopausal status and measurement indices, a REM meta-analysis was performed again using the ES for each dataset to calculate a summary effect size (sES) and its 95% CI. Meta-analysis was performed only in cases in which two or more datasets were found in the subgroup analyses. In the meta-analysis, heterogeneity was assessed by I-squared values (%).
- Furthermore, to investigate the dose-response relationship in breast cancer risk in accordance with the density level presented in breast density indices, a random effects dose-response meta-regression (DRMR) was conducted [27]. The median values within the interval were used for dosage determination, and the lower limit was set at zero when the lowest interval was open. When the highest interval was open, the median interval of the adjacent interval was used. If the density index was PD, the dosage unit was determined to be 25%. The statistical significance level was set at 5%, and Stata version 14.0 (StataCorp, College Station, TX, USA) was used.
METHODS
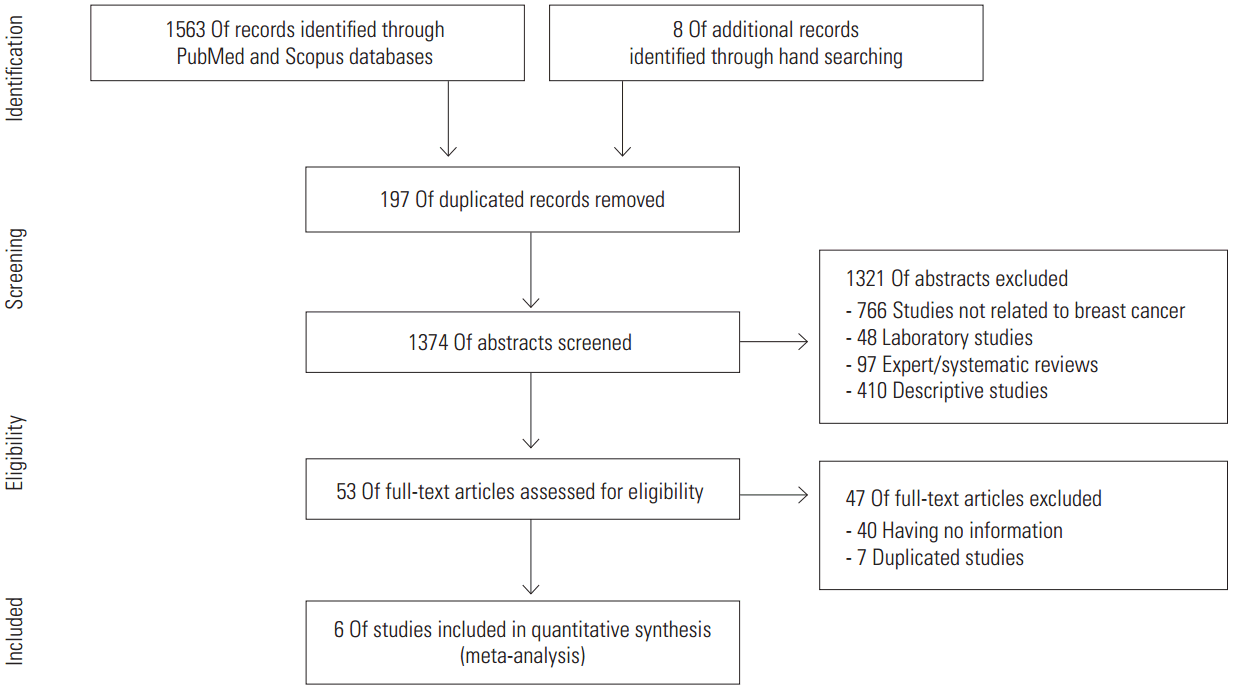
- Figure 1 shows a flow diagram illustrating a series of processes from the searching, screening, and evaluation stages to the final selection of articles to include in the analysis. From the two databases, PubMed and Scopus, a list of 1563 articles was obtained by applying the search formula. Eight articles acquired from the hand-searching process were added to the list, and then 197 duplicates were removed, leaving a list of 1374 articles. From this list, 1321 articles were eliminated based on abstracts and titles. The texts of the 53 remaining articles were obtained and the content was evaluated to remove 47 articles, leaving the final six articles for meta-analysis [20-22,28-30].
- The exclusion criteria during the selection process were the following: (1) 766 studies were not related to breast cancer; (2) 48 articles were laboratory studies; (3) 97 studies were expert or SRs; (4) 410 articles were descriptive epidemiological studies, including cross-sectional prevalence studies; (5) 40 analytical epidemiology studies did not provide sufficient information for a meta-analysis; and (6) seven articles were duplicate studies. The study subjects of Nagata et al. [21] were patients at Gifu City Hospital, and four duplicates were removed [31-34]. The study subjects of Lee et al. [30] were participants of the Singapore Breast Cancer Screening Programme (SBCSP), and two duplicates were removed [23,35]. The study subjects of Kim et al. [20] were patients at the Samsung Medical Cancer in Korea, and one duplicate was removed [36].
- Table 1 shows a summary of the final six articles with 17 datasets based on type of breast density index and menopausal status. Categorized by country, the six articles included three Japanese studies, two Korean studies, and one Singaporean study. One was a cohort study and five were case-control studies. Seven datasets were of premenopausal women and eight were of postmenopausal women. In terms of the breast density index, five used PD, four used TBA, and two or fewer datasets used the remaining indices.
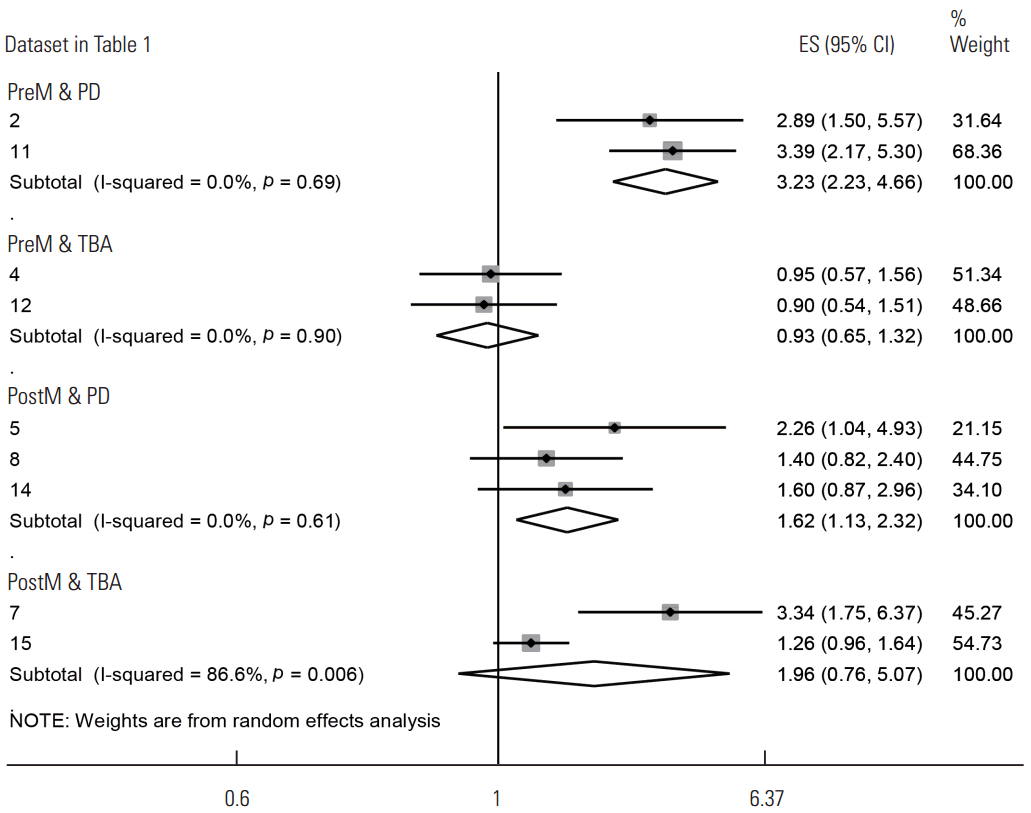
- Since the findings varied in accordance with the index type and menopausal status, subgroup analysis was performed rather than calculating the sES values of the 17 datasets in order to control for potential heterogeneity. In other words, the datasets were divided according to menopausal status, and meta-analysis was performed only in cases with two or more datasets for each density index (Table 2, Figure 2). In premenopausal women, the PD index was significantly associated with elevated breast cancer risk (sES, 3.23; 95% CI, 2.23 to 4.66; I2=0.0%), whereas the TBA index did not show a statistically significant association. In the group of postmenopausal women, the TBA index did not show a statistically significant association, whereas the PD index was associated with a significant increase in breast cancer risk (sES, 1.62; 95% CI, 1.13 to 2.32; I2=0.0%). For premenopausal and postmenopausal women, meta-analysis was performed if two or more datasets were present for each density index, and the results showed significantly elevated breast cancer risks for the PD, density area, and volumetric density indices.
- Among the six articles selected, three provided the information necessary for DRMR analysis [21,22,29]. Three datasets (2, 5, and 8 in Table 1) were obtained using the PD index, and homogeneity was detected between two datasets (5 and 8 in Table 1) of postmenopausal women (p=0.35), showing a risk increase of 1.73 times for each 25% increase in PD in postmenopausal women (95% CI, 1.20 to 2.47).
RESULTS
- In this study, the first SR of breast density and breast cancer risk in Asian women, breast cancer risk was found to increase as the PD value increased. Although the TBA index did not show statistical significance, the risk increased by 73% for each 25% increase in PD in postmenopausal women, which indicates that higher breast cancer risk is associated with higher PD values in women in Asian countries.
- However, the risk calculated for PD in premenopausal and postmenopausal women was estimated to be 2.21 times that of baseline (95% CI, 1.52 to 3.21), which is lower than the risk elevation of four to six times that has been confirmed in Western women (two to four). Four factors may account for this discrepancy. First, breast density itself is not a risk factor, but a phenomenon determined by other risk factors [37-40]. Density can be affected by obesity, family history, genotype as well as obstetrical history [10]. Second, in Asian women, the positive predictive value in breast cancer diagnosis decreases with decreased sensitivity in mammography of denser breasts, resulting in underestimation of cancer occurrence [41,42]. Third, risk levels were found to change in accordance with the type of density measurement index [43-45], suggesting that different measurement indices have been used by different researchers [2,9], and that different indices may be appropriate depending on race [16]. Fourth, this study analyzed limited data, meaning that the conclusions may be tentative. Further studies evaluating breast density, as measured using several indices, and the risk of breast cancer in Asian women are needed.
- The limitations of the meta-analysis conducted in this study include the following. First, an overall ES reflecting information from all 6 articles was not calculated, not only because the number of articles related to Asian women was small, but also because the breast density index varied across articles. However, the study performed by Wong et al. [23], which was excluded because its participants overlapped with the SBCSP participants, presented breast cancer risks adjusted for menopausal status and the PD index. When this was added to the five data-sets using PD in the meta-analysis, as shown in Table 2, the sES increased from 2.12 to 2.30 (95% CI, 1.67 to 3.16; I2=40.5%, not shown in Table 2). This fact underscores the necessity of further studies. Second, the subgroup analysis was performed imperfectly. When two groups (premenopausal and postmenopausal women) were differentiated in a single article, it was possible that the sES values were determined by calculations within the subgroup analysis. However, the studies of Nagao et al. [28] and Lee et al. [30] could not be used in the subgroup analysis because the results were not divided according to menopausal status. Furthermore, in cases where various indices were used for the same subjects, these results could not be incorporated without omission, resulting in a selective analysis within subgroups as well. Third, the analysis of premenopausal women was insufficient for DRMR. Applying DRMR to the density indices was possible because three datasets were established from the total of two articles on Japanese women, while only one was possible for premenopausal women. The aforementioned three limitations could be overcome by creating a single database for a pooled analysis of the selected articles.
- The limitations regarding breast density in mammography include the following. First, the subjects included only women who were born and lived in Asia. In other words, women who were born in Asia but emigrated overseas were excluded. This decision was based on studies reporting that immigration as an environmental change affects breast cancer risk [45]. In the future, studies will be needed to investigate how breast density affects cancer risk among people of the same race depending on emigration [46]. Second, in the five case-control studies, the most recent mammography results before breast cancer diagnosis were used as breast density values. This design does not reflect the fact that breast density changes with age in individual women [46-48]. In the future, cohort studies that investigate breast cancer risk according to individual changes in breast density will be needed.
DISCUSSION
- In conclusion, regardless of menopausal status, breast cancer risk in Asian women increased with breast density measured using PD. In particular, postmenopausal women with a high PD index had an elevated risk of breast cancer. As this SR suggests that the PD index represents breast cancer risks well in Asian women, we propose the further development of a breast-cancer risk prediction model involving PD for Asian women. To investigate the risks more precisely, a pooled analysis is proposed, along with the application of PD in breast cancer prediction models in Asian women.
CONCLUSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
Notes
| Dataset | First author (year of publication) [reference number] | Region | Source of subjects | Menopausal status | Ratio of cases to controls | Index | Intervals | aOR | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Nagao (2003) [28] | Japan | Gihoku General Hospital | 237:742 | Wolfe | P1 | 1.03 | 0.69, 1.55 | |
| P2 | 0.68 | 0.36, 1.31 | |||||||
| DY | 2.20 | 1.02, 4.77 | |||||||
| 2 | Nagata (2005) [21] | Japan | Gifu Hospital | PreM | 71:370 | PD | 1-24 | 2.27 | 0.64, 8.08 |
| 25-49 | 4.01 | 1.16, 13.9 | |||||||
| 50-75 | 4.37 | 1.24, 15.4 | |||||||
| 75+ | 1.36 | 0.31, 6.60 | |||||||
| 3 | DA | 0.1-12.0 | 1.58 | 0.41, 6.23 | |||||
| 12.1-26.3 | 4.03 | 1.14, 14.2 | |||||||
| 26.4-44.4 | 5.14 | 1.45, 18.3 | |||||||
| 44.5+ | 2.78 | 0.77, 10.1 | |||||||
| 4 | TBA | 52.3-66.0 | 0.66 | 0.28, 1.56 | |||||
| 66.1-83.8 | 0.85 | 0.36, 2.04 | |||||||
| 83.9+ | 1.53 | 0.64, 3.65 | |||||||
| 5 | PostM | 75:289 | PD | 1-24 | 1.17 | 0.55, 2.49 | |||
| 25-49 | 3.00 | 1.20, 7.48 | |||||||
| 50+ | 4.19 | 1.33, 13.2 | |||||||
| 6 | DA | 0.1-9.5 | 0.83 | 0.33, 2.12 | |||||
| 9.6-21.3 | 1.07 | 0.41, 2.80 | |||||||
| 21.4+ | 4.02 | 1.80, 8.94 | |||||||
| 7 | TBA | 57.7-73.7 | 1.89 | 0.61, 5.91 | |||||
| 73.8-97.0 | 4.15 | 1.39, 12.4 | |||||||
| 97.1+ | 4.65 | 1.50, 14.4 | |||||||
| 8 | Kotsuma (2008) [22] | Japan | Osaka University 1999-2003 | PostM | 205:223 | PD | 3.4-8.8 | 0.98 | 0.51, 1.91 |
| 8.9-16.5 | 0.94 | 0.48, 1.84 | |||||||
| 16.6-28.7 | 1.36 | 0.70, 2.65 | |||||||
| 28.8- | 3.02 | 1.58, 5.77 | |||||||
| 9 | Park (2014) [29] | Korea | National Cancer Center | PostM | 302:774 | VDG | 8.0-15.0 | 2.64 | 1.85, 3.78 |
| 15.1+ | 3.07 | 1.89, 4.99 | |||||||
| 10 | PreM | 374:435 | 8.0-15.0 | 1.87 | 0.91, 3.86 | ||||
| 15.1+ | 2.05 | 0.99, 4.23 | |||||||
| 11 | Kim (2015) [20] | Korea | Samsung Medical Center | PreM | 134:395 | PD | 5-9 | 2.46 | 0.52, 11.52 |
| 10-24 | 3.04 | 0.71, 12.96 | |||||||
| 25-49 | 4.08 | 0.93, 17.82 | |||||||
| 50+ | 5.73 | 0.93, 35.40 | |||||||
| 12 | TBA | Q2 | 0.70 | 0.43, 1.14 | |||||
| Q3 | 1.07 | 0.67, 1.73 | |||||||
| Q4 | 0.97 | 0.57, 1.67 | |||||||
| 13 | ADA | Q2 | 1.50 | 0.72, 3.12 | |||||
| Q3 | 1.56 | 0.77, 3.17 | |||||||
| Q4 | 1.99 | 1.00, 3.97 | |||||||
| 14 | PostM | 79:235 | PD | 5-9 | 1.11 | 0.58, 2.10 | |||
| 10-24 | 1.05 | 0.54, 2.06 | |||||||
| 25-49 | 1.40 | 0.48, 4.08 | |||||||
| 50+ | 3.96 | 1.38, 40.87 | |||||||
| 15 | TBA | Q2 | 1.20 | 0.53, 2.70 | |||||
| Q3 | 1.26 | 0.57, 2.79 | |||||||
| Q4 | 1.52 | 0.64, 3.57 | |||||||
| 16 | ADA | Q2 | 0.88 | 0.47, 1.62 | |||||
| Q3 | 0.78 | 0.36, 1.67 | |||||||
| Q4 | 1.55 | 0.78, 3.06 | |||||||
| 17 | Lee (2015) [30] | Singapore | Singapore Breast Cancer | (17 y follow-up) | 680:23 481 | MDA | 11-20 | 1.60 | 1.22, 2.10 |
| Screening Programme | 21-30 | 2.20 | 1.65, 2.92 | ||||||
| 31-40 | 2.33 | 1.71, 3.20 | |||||||
| 41-50 | 2.12 | 1.43, 3.14 | |||||||
| 51-60 | 3.27 | 2.24, 4.76 |
aOR, adjusted odds ratio; CI, confidence interval; PreM, premenopausal; PostM, postmenopausal; Wolfe, Wolfe classification; PD, percent density (%); DA, density area (cm2); MDA, mean dense area (cm2); TBA, total breast area (cm2); VDG, volumetric density grade (%); ADA, absolute dense area (cm2).
1 A case-control study design was used for all results except the 17th dataset, which was obtained from a prospective cohort study.
| Menopausal status | Index | Dataset in Table 1 | sES | 95% CI | I2 |
|---|---|---|---|---|---|
| PreM | PD | 2, 11 | 3.23 | 2.23, 4.66 | 0.0 |
| TBA | 4, 12 | 0.93 | 0.65, 1.32 | 0.0 | |
| PD + VDG | 2, 10, 11 | 2.74 | 1.95, 3.85 | 16.2 | |
| TBA + VDG | 4, 10, 12 | 1.17 | 0.73, 1.87 | 58.8 | |
| PostM | PD | 5, 8, 14 | 1.62 | 1.13, 2.32 | 0.0 |
| TBA | 7, 15 | 1.96 | 0.76, 5.07 | 86.6 | |
| PD +VDG | 5, 8, 9, 14 | 2.02 | 1.39, 2.95 | 52.3 | |
| TBA +VDG | 7, 9, 15 | 2.19 | 1.16, 4.14 | 89.4 | |
| PreM and PostM | PD | 2, 5, 8, 11, 14 | 2.21 | 1.52, 3.21 | 50.0 |
| TBA | 4, 7, 12, 15 | 1.32 | 0.84, 2.08 | 74.1 | |
| DA | 3, 6 | 2.49 | 1.30, 4.78 | 24.2 | |
| VDG | 9, 10 | 2.52 | 1.84, 3.46 | 21.6 | |
| ADA | 13, 16 | 1.24 | 0.72, 2.15 | 35.7 | |
| Not distinguished | Wolf +MDA | 1, 17 | 1.71 | 0.79, 3.68 | 83.7 |
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87-108ArticlePubMed
- 2. Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res 2011;13(5):R100ArticlePubMedPMC
- 3. Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 2008;10(1):201ArticlePubMedPMC
- 4. Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011;13(6):223ArticlePubMedPMC
- 5. Saftlas AF, Szklo M. Mammographic parenchymal patterns and breast cancer risk. Epidemiol Rev 1987;9: 146-174PubMed
- 6. Warner E, Lockwood G, Tritchler D, Boyd NF. The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. Cancer Detect Prev 1992;16(1):67-72PubMed
- 7. Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev 1993;15(1):196-208ArticlePubMed
- 8. Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 1998;7(12):1133-1144ArticlePubMed
- 9. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15(6):1159-1169ArticlePubMed
- 10. Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Mammographic density and breast cancer risk in white and African American women. Breast Cancer Res Treat 2012;135(2):571-580ArticlePubMedPMC
- 11. Habel LA, Capra AM, Oestreicher N, Greendale GA, Cauley JA, Bromberger J, et al. Mammographic density in a multiethnic cohort. Menopause 2007;14(5):891-899ArticlePubMed
- 12. del Carmen MG, Hughes KS, Halpern E, Rafferty E, Kopans D, Parisky YR, et al. Racial differences in mammographic breast density. Cancer 2003;98(3):590-596ArticlePubMed
- 13. El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol 2001;11(4):257-263ArticlePubMed
- 14. Tan SM, Evans AJ, Lam TP, Cheung KL. How relevant is breast cancer screening in the Asia/Pacific region? Breast 2007;16(2):113-119ArticlePubMed
- 15. Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol 2001;30(5):959-965ArticlePubMed
- 16. Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol 2004;159(2):140-147ArticlePubMed
- 17. Kawahara M, Sato S, Ida Y, Watanabe M, Fujishima M, Ishii H, et al. Factors influencing breast density in Japanese women aged 40-49 in breast cancer screening mammography. Acta Med Okayama 2013;67(4):213-217PubMed
- 18. Bae JM. Two hypotheses of dense breasts and viral infection for explaining incidence of breast cancer by age group in Korean women. Epidemiol Health 2014;36: e2014020Article
- 19. Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control 2010;21(11):1777-1785ArticlePubMed
- 20. Kim BK, Choi YH, Nguyen TL, Nam SJ, Lee JE, Hopper JL, et al. Mammographic density and risk of breast cancer in Korean women. Eur J Cancer Prev 2015;24(5):422-429ArticlePubMed
- 21. Nagata C, Matsubara T, Fujita H, Nagao Y, Shibuya C, Kashiki Y, et al. Mammographic density and the risk of breast cancer in Japanese women. Br J Cancer 2005;92(12):2102-2106ArticlePubMedPMC
- 22. Kotsuma Y, Tamaki Y, Nishimura T, Tsubai M, Ueda S, Shimazu K, et al. Quantitative assessment of mammographic density and breast cancer risk for Japanese women. Breast 2008;17(1):27-35ArticlePubMed
- 23. Wong CS, Lim GH, Gao F, Jakes RW, Offman J, Chia KS, et al. Mammographic density and its interaction with other breast cancer risk factors in an Asian population. Br J Cancer 2011;104(5):871-874ArticlePubMedPMC
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339: b2700ArticlePubMedPMC
- 25. Bae JM, Kim EH. Citation discovery tools for conducting adaptive meta-analyses to update systematic reviews. J Prev Med Public Health 2016;49(2):129-133ArticlePubMedPMCPDF
- 26. Bae JM. Comparison of methods of extracting information for meta-analysis of observational studies in nutritional epidemiology. Epidemiol Health 2016;38: e2016003Article
- 27. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6(1):40-57ArticlePDF
- 28. Nagao Y, Kawaguchi Y, Sugiyama Y, Saji S, Kashiki Y. Relationship between mammographic density and the risk of breast cancer in Japanese women: a case-control study. Breast Cancer 2003;10(3):228-233ArticlePubMed
- 29. Park IH, Ko K, Joo J, Park B, Jung SY, Lee S, et al. High volumetric breast density predicts risk for breast cancer in postmenopausal, but not premenopausal, Korean women. Ann Surg Oncol 2014;21(13):4124-4132ArticlePubMed
- 30. Lee CP, Choi H, Soo KC, Tan MH, Chay WY, Chia KS, et al. Mammographic breast density and common genetic variants in breast cancer risk prediction. PLoS One 2015;10(9):e0136650Article
- 31. Woolcott CG, Koga K, Conroy SM, Byrne C, Nagata C, Ursin G, et al. Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case-control studies. Breast Cancer Res Treat 2012;132(3):1163-1171ArticlePubMedPMC
- 32. Conroy SM, Woolcott CG, Koga KR, Byrne C, Nagata C, Ursin G, et al. Mammographic density and risk of breast cancer by adiposity: an analysis of four case-control studies. Int J Cancer 2012;130(8):1915-1924ArticlePubMed
- 33. Conroy SM, Koga K, Woolcott CG, Dahl T, Byrne C, Nagata C, et al. Higher alcohol intake may modify the association between mammographic density and breast cancer: an analysis of three case-control studies. Cancer Epidemiol 2012;36(5):458-460ArticlePubMedPMC
- 34. Maskarinec G, Nakamura KL, Woolcott CG, Conroy SM, Byrne C, Nagata C, et al. Mammographic density and breast cancer risk by family history in women of white and Asian ancestry. Cancer Causes Control 2015;26(4):621-626ArticlePubMedPMC
- 35. Jakes RW, Duffy SW, Ng FC, Gao F, Ng EH. Mammographic parenchymal patterns and risk of breast cancer at and after a prevalence screen in Singaporean women. Int J Epidemiol 2000;29(1):11-19ArticlePubMed
- 36. Nguyen TL, Aung YK, Evans CF, Yoon-Ho C, Jenkins MA5, Sung J, et al. Mammographic density defined by higher than conventional brightness threshold better predicts breast cancer risk for full-field digital mammograms. Breast Cancer Res 2015;17: 142ArticlePubMedPMC
- 37. Lai CW, Law HK. Mammographic breast density in chinese women: spatial distribution and autocorrelation patterns. PLoS One 2015;10(9):e0136881Article
- 38. Jeon JH, Kang JH, Kim Y, Lee HY, Choi KS, Jun JK, et al. Reproductive and hormonal factors associated with fatty or dense breast patterns among Korean women. Cancer Res Treat 2011;43(1):42-48ArticlePubMedPMCPDF
- 39. Dite GS, Gurrin LC, Byrnes GB, Stone J, Gunasekara A, McCredie MR, et al. Predictors of mammographic density: insights gained from a novel regression analysis of a twin study. Cancer Epidemiol Biomarkers Prev 2008;17(12):3474-3481ArticlePubMedPMC
- 40. Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 2005;6(10):798-808ArticlePubMed
- 41. Machida Y, Tozaki M, Shimauchi A, Yoshida T. Breast density: the trend in breast cancer screening. Breast Cancer 2015;22(3):253-261ArticlePubMed
- 42. Tan YY, Wee SB, Tan MP, Chong BK. Positive predictive value of BI-RADS categorization in an Asian population. Asian J Surg 2004;27(3):186-191ArticlePubMed
- 43. Colin C, Prince V, Valette PJ. Can mammographic assessments lead to consider density as a risk factor for breast cancer? Eur J Radiol 2013;82(3):404-411ArticlePubMed
- 44. Kopans DB. Basic physics and doubts about relationship between mammographically determined tissue density and breast cancer risk. Radiology 2008;246(2):348-353ArticlePubMed
- 45. Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev 2003;12(4):332-338PubMed
- 46. Maskarinec G, Pagano I, Chen Z, Nagata C, Gram IT. Ethnic and geographic differences in mammographic density and their association with breast cancer incidence. Breast Cancer Res Treat 2007;104(1):47-56ArticlePubMed
- 47. Bae JM, Shin SY, Kim EH, Kim YN, Nam CM. Distribution of dense breasts using screening mammography in Korean women: a retrospective observational study. Epidemiol Health 2014;36: e2014027Article
- 48. Kim EH, Bae JM. Potential availability of dense mammogrphy for prevention of breast cancer in Korean women. Korean J Fam Pract 2014;4(3):181-185. (Korean)
REFERENCES
Figure & Data
References
Citations

- Intelligent scoring system based on dynamic optical breast imaging for early detection of breast cancer
Yaoyao Li, Yipei Zhang, Qiang Yu, Chenglong He, Xiguo Yuan
Biomedical Optics Express.2024; 15(3): 1515. CrossRef - Physical Activity and Mammographic Density in Japanese Women
Mihye Lee, Rina Kotake, Hideko Yamauchi
Cancer Epidemiology, Biomarkers & Prevention.2024; 33(3): 365. CrossRef - The association between mammographic density and breast cancer risk in Chinese women: a systematic review and meta-analysis
Song Bai, Di Song, Ming Chen, Xiaoshu Lai, Jinfeng Xu, Fajin Dong
BMC Women's Health.2024;[Epub] CrossRef - Polygenic risk score-based prediction of breast cancer risk in Taiwanese women with dense breast using a retrospective cohort study
Chih-Chiang Hung, Sin-Hua Moi, Hsin-I Huang, Tzu-Hung Hsiao, Chi-Cheng Huang
Scientific Reports.2024;[Epub] CrossRef - Polygenic risk scores for prediction of breast cancer in Korean women
Yon Ho Jee, Weang-Kee Ho, Sohee Park, Douglas F Easton, Soo-Hwang Teo, Keum Ji Jung, Peter Kraft
International Journal of Epidemiology.2023; 52(3): 796. CrossRef - Estimating Age-Specific Mean Sojourn Time of Breast Cancer and Sensitivity of Mammographic Screening by Breast Density among Korean Women
Eunji Choi, Mina Suh, So-Youn Jung, Kyu-Won Jung, Sohee Park, Jae Kwan Jun, Kui Son Choi
Cancer Research and Treatment.2023; 55(1): 136. CrossRef - Current Trends in the Utilization of Preoperative Breast Magnetic Resonance Imaging Among Women With Newly Diagnosed Breast Cancer
I-Wen Pan, Tina W.F. Yen, Isabelle Bedrosian, Ya-Chen Tina Shih
JCO Oncology Practice.2023; 19(7): 446. CrossRef - International Interobserver Variability of Breast Density Assessment
Leah H. Portnow, Lina Choridah, Kardinah Kardinah, Triwulan Handarini, Ruud Pijnappel, Adriana M.J. Bluekens, Lucien E.M. Duijm, Peter K. Schoub, Pamela S. Smilg, Liat Malek, Jessica W.T. Leung, Sughra Raza
Journal of the American College of Radiology.2023; 20(7): 671. CrossRef - Deep Learning Analysis of Mammography for Breast Cancer Risk Prediction in Asian Women
Hayoung Kim, Jihe Lim, Hyug-Gi Kim, Yunji Lim, Bo Kyoung Seo, Min Sun Bae
Diagnostics.2023; 13(13): 2247. CrossRef - The diagnostic accuracy of mammography and ultrasonography for recurrent breast cancer after breast conserving treatment
Piyakan Pathanasethpong, Supajit Nawapun, Payia Chadbunchachai, Ongart Somintara, Chaiwat Apivatanasiri, Arunnit Boonrod
European Journal of Radiology Open.2023; 11: 100514. CrossRef - Fine-needle aspiration biopsy possibilities in studying the molecular genetic landscape of breast tissue
V. V. Rodionov, O. V. Burmenskaya, V. V. Kometova, A. A. Smetnik, M. V. Rodionova, D. Yu. Trofimov, L. A. Ashrafyan, G. T. Sukhikh
Tumors of female reproductive system.2023; 19(4): 16. CrossRef - miR-146a Enhances the Sensitivity of Breast Cancer Cells to Paclitaxel by Downregulating IRAK1
Yalun Li, Weilong Li, Jun Lin, Chunjing Lv, Guangdong Qiao
Cancer Biotherapy and Radiopharmaceuticals.2022; 37(8): 624. CrossRef - Current status of AYA-generation breast cancer: trends worldwide and in Japan
Manabu Futamura, Kazuhiro Yoshida
International Journal of Clinical Oncology.2022; 27(1): 16. CrossRef - A Machine Learning Ensemble Based on Radiomics to Predict BI-RADS Category and Reduce the Biopsy Rate of Ultrasound-Detected Suspicious Breast Masses
Matteo Interlenghi, Christian Salvatore, Veronica Magni, Gabriele Caldara, Elia Schiavon, Andrea Cozzi, Simone Schiaffino, Luca Alessandro Carbonaro, Isabella Castiglioni, Francesco Sardanelli
Diagnostics.2022; 12(1): 187. CrossRef - Global guidelines for breast cancer screening: A systematic review
Wenhui Ren, Mingyang Chen, Youlin Qiao, Fanghui Zhao
The Breast.2022; 64: 85. CrossRef - Utility of U-Net for the objective segmentation of the fibroglandular tissue region on clinical digital mammograms
Mika Yamamuro, Yoshiyuki Asai, Naomi Hashimoto, Nao Yasuda, Hiorto Kimura, Takahiro Yamada, Mitsutaka Nemoto, Yuichi Kimura, Hisashi Handa, Hisashi Yoshida, Koji Abe, Masahiro Tada, Hitoshi Habe, Takashi Nagaoka, Seiun Nin, Kazunari Ishii, Yohan Kondo
Biomedical Physics & Engineering Express.2022; 8(4): 045016. CrossRef - Breast Cancer Disparities in Asian Women: The Need for Disaggregated Research
Lauren Fane, Tithi Biswas, Charulata Jindal, Yuk Ming Choi, Jimmy T. Efird
International Journal of Environmental Research and Public Health.2022; 19(16): 9790. CrossRef - Vertical Breast Displacement in Asian Women During Exercise: influence of Bra Type, Size and Different Parts of the Breast
Xinyang Sheng, Xiaona Chen, Mark John Lake
Fibres & Textiles in Eastern Europe.2022; 30(3): 1. CrossRef - Studies of parenchymal texture added to mammographic breast density and risk of breast cancer: a systematic review of the methods used in the literature
Akila Anandarajah, Yongzhen Chen, Graham A. Colditz, Angela Hardi, Carolyn Stoll, Shu Jiang
Breast Cancer Research.2022;[Epub] CrossRef - Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach
William Lotter, Abdul Rahman Diab, Bryan Haslam, Jiye G. Kim, Giorgia Grisot, Eric Wu, Kevin Wu, Jorge Onieva Onieva, Yun Boyer, Jerrold L. Boxerman, Meiyun Wang, Mack Bandler, Gopal R. Vijayaraghavan, A. Gregory Sorensen
Nature Medicine.2021; 27(2): 244. CrossRef - Association between changes in mammographic density category and the risk of breast cancer: A nationwide cohort study in East‐Asian women
Soyeoun Kim, Boyoung Park
International Journal of Cancer.2021; 148(11): 2674. CrossRef - Dense Breast Notification Laws’ Association With Outcomes in the US Population: A Cross-Sectional Study
Nancy R. Kressin, Tracy A. Battaglia, Jolie B. Wormwood, Priscilla J. Slanetz, Christine M. Gunn
Journal of the American College of Radiology.2021; 18(5): 685. CrossRef - Breast Cancer Lesion Detection and Classification in mammograms using Deep Neural
A R J Silalahi
IOP Conference Series: Materials Science and Engineering.2021; 1115(1): 012018. CrossRef - Cancer Progress and Priorities: Breast Cancer
Serena C. Houghton, Susan E. Hankinson
Cancer Epidemiology, Biomarkers & Prevention.2021; 30(5): 822. CrossRef - Enhancing the Screening Efficiency of Breast Cancer by Combining Conventional Medical Imaging Examinations With Circulating Tumor Cells
Yang Gao, Wan-Hung Fan, Chaohui Duan, Wenhe Zhao, Jun Zhang, Xixiong Kang
Frontiers in Oncology.2021;[Epub] CrossRef - Comparison of the diagnostic performances of circulating tumor cells and the serum tumor markers CEA, CA125, and CA15-3 for breast cancer: a retrospective case-control study
Yi Luan, Jie Wei, Ke Wang, Donghao Cai, Xiaohong Luo, Wanhung Fan, Haijiang Wang, Chaohui Duan
Journal of Bio-X Research.2021; 4(2): 60. CrossRef - Meme kanseri olan Türk kadın hastalarda meme dansitesinin klinik ve patolojik bulgularla ilişkileri
Nihan TURHAN, Dilek YILMAZ, Levent YEŞİLYURT
Pamukkale Medical Journal.2021;[Epub] CrossRef - Evidence and assessment of parenchymal patterns of ultrasonography for breast cancer detection among Chinese women: a cross-sectional study
Zhongtao Bao, Yanchun Zhao, Shuqiang Chen, Xiaoyu Chen, Xiang Xu, Linglin Wei, Ling Chen
BMC Medical Imaging.2021;[Epub] CrossRef - The Relationship Between Breast Density Change During Menopause and the Risk of Breast Cancer in Korean Women
Danbee Kang, Ji-Yeon Kim, Ji-Young Kim, Han Song Mun, Sook Ja Yoon, Jieun Lee, Gayeon Han, Young-Hyuck Im, Soo-Young Shin, Se Kyung Lee, Jong-Han Yu, Kyung-Hyun Lee, Mincheol Kim, Dohyun Park, Yoon-Ho Choi, Ok Soon Jeong, Jean Hyoung Lee, Se Yong Jekal, J
Cancer Prevention Research.2021; 14(12): 1119. CrossRef - Feasibility of Portable Microwave Imaging Device for Breast Cancer Detection
Mio Adachi, Tsuyoshi Nakagawa, Tomoyuki Fujioka, Mio Mori, Kazunori Kubota, Goshi Oda, Takamaro Kikkawa
Diagnostics.2021; 12(1): 27. CrossRef - Primary prevention of breast cancer
V.F. Levshin
Profilakticheskaya meditsina.2021; 24(11): 117. CrossRef - Immigration history, lifestyle characteristics, and breast density in the Vietnamese American Women’s Health Study: a cross-sectional analysis
Eunjung Lee, Namphuong Doanvo, MiHee Lee, Zayar Soe, Alice W. Lee, Cam Van Doan, Dennis Deapen, Giske Ursin, Darcy Spicer, Peggy Reynolds, Anna H. Wu
Cancer Causes & Control.2020; 31(2): 127. CrossRef - Long-Term Outcomes of Immediate Autologous Breast Reconstruction for Breast Cancer Patients
Akimitsu Yamada, Kazutaka Narui, Toshihiko Satake, Shoko Adachi, Mikiko Tanabe, Daisuke Shimizu, Takashi Ishikawa, Itaru Endo
Journal of Surgical Research.2020; 251: 78. CrossRef - Density of breast: An independent risk factor for developing breast cancer, a prospective study at two premium breast centers
Chia Hwee Lo, Xin Ying Chai, Shirley Shy Wen Ting, Sze Chao Ang, Xinlin Chin, Lay Teng Tan, Peeroo Saania, Tuan Nur' Azmah Tuan Mat, Seniyah Mat Sikin, Anil Gandhi
Cancer Medicine.2020; 9(9): 3244. CrossRef - A Review of Breast Density Implications and Breast Cancer Screening
Jingge Lian, Kangan Li
Clinical Breast Cancer.2020; 20(4): 283. CrossRef - Breast Cancer Incidence Trends by Estrogen Receptor Status Among Asian American Ethnic Groups, 1990–2014
Alyssa W Tuan, Brittny C Davis Lynn, Pavel Chernyavskiy, Mandi Yu, Scarlett L Gomez, Gretchen L Gierach, Philip S Rosenberg
JNCI Cancer Spectrum.2020;[Epub] CrossRef - Supplemental breast cancer-screening ultrasonography in women with dense breasts: a systematic review and meta-analysis
Wei-Hsin Yuan, Hui-Chen Hsu, Ying-Yuan Chen, Chia-Hung Wu
British Journal of Cancer.2020; 123(4): 673. CrossRef - Mammographic breast density, its changes, and breast cancer risk in premenopausal and postmenopausal women
Eun Young Kim, Yoosoo Chang, Jiin Ahn, Ji‐Sup Yun, Yong Lai Park, Chan Heun Park, Hocheol Shin, Seungho Ryu
Cancer.2020; 126(21): 4687. CrossRef - Evaluation of automated volumetric breast density software in comparison with visual assessments in an Asian population
Kartini Rahmat, Nazimah Ab Mumin, Marlina Tanty Ramli Hamid, Farhana Fadzli, Wei Lin Ng, Nadia Fareeda Muhammad Gowdh
Medicine.2020; 99(39): e22405. CrossRef - Prevalence of Women with Dense Breasts in Korea: Results from a Nationwide Cross-sectional Study
Hye-Mi Jo, Eun Hye Lee, Kyungran Ko, Bong Joo Kang, Joo Hee Cha, Ann Yi, Hae Kyoung Jung, Jae Kwan Jun
Cancer Research and Treatment.2019; 51(4): 1295. CrossRef - Methodological Challenges and Updated Findings from a Meta-analysis of the Association between Mammographic Density and Breast Cancer
Daniela Bond-Smith, Jennifer Stone
Cancer Epidemiology, Biomarkers & Prevention.2019; 28(1): 22. CrossRef - The role of breast tomosynthesis in a predominantly dense breast population at a tertiary breast centre: breast density assessment and diagnostic performance in comparison with MRI
Daniel Förnvik, Masako Kataoka, Mami Iima, Akane Ohashi, Shotaro Kanao, Masakazu Toi, Kaori Togashi
European Radiology.2018; 28(8): 3194. CrossRef - Breast-density assessment with hand-held ultrasound: A novel biomarker to assess breast cancer risk and to tailor screening?
Sergio J. Sanabria, Orcun Goksel, Katharina Martini, Serafino Forte, Thomas Frauenfelder, Rahel A. Kubik-Huch, Marga B. Rominger
European Radiology.2018; 28(8): 3165. CrossRef - The role of matricellular proteins and tissue stiffness in breast cancer: a systematic review
Sirio Fiorino, Salomone Di Saverio, Paolo Leandri, Andrea Tura, Chiara Birtolo, Mauro Silingardi, Dario de Biase, Eli Avisar
Future Oncology.2018; 14(16): 1601. CrossRef - Molecular mechanisms of the preventable causes of cancer in the United States
Erica A. Golemis, Paul Scheet, Tim N. Beck, Eward M. Scolnick, David J. Hunter, Ernest Hawk, Nancy Hopkins
Genes & Development.2018; 32(13-14): 868. CrossRef - Inactivated Sendai virus particle upregulates cancer cell expression of intercellular adhesion molecule‐1 and enhances natural killer cell sensitivity on cancer cells
Simin Li, Tomoyuki Nishikawa, Yasufumi Kaneda
Cancer Science.2017; 108(12): 2333. CrossRef
 KSPM
KSPM


 PubReader
PubReader ePub Link
ePub Link Cite
Cite