Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 56(3); 2023 > Article
-
Original Article
COVID-19 Vaccination and Clinical Outcomes at a Secondary Referral Hospital During the Delta Variant-dominant Period in West Sumatra, Indonesia -
Didan Ariadapa Rahadi1
 , Elfira Yusri2, Syandrez Prima Putra3,4
, Elfira Yusri2, Syandrez Prima Putra3,4 , Rima Semiarty5,6
, Rima Semiarty5,6 , Dian Pertiwi2, Cimi Ilmiawati6,7
, Dian Pertiwi2, Cimi Ilmiawati6,7
-
Journal of Preventive Medicine and Public Health 2023;56(3):221-230.
DOI: https://doi.org/10.3961/jpmph.23.077
Published online: May 31, 2023
1Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
2Department of Clinical Pathology, Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
3Department of Microbiology, Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
4Center for Infectious Disease Diagnostic and Research (PDRPI), Faculty of Medicine, Universitas Andalas, Padang, Indonesia
5Department of Public Health, Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
6Doctoral Program of Public Health, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
7Department of Pharmacology, Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
- Corresponding author: Cimi Ilmiawati, Department of Pharmacology, Undergraduate Program of Medicine, Faculty of Medicine, Universitas Andalas, Kampus Limau Manis, Gedung B Lt.1, Limau Manis, Padang 25166, Indonesia E-mail: ilmiawati@med.unand.ac.id
Copyright © 2023 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- The second wave of coronavirus disease 2019 (COVID-19) cases in Indonesia, during which the Delta variant predominated, took place after a vaccination program had been initiated in the country. This study was conducted to assess the impact of COVID-19 vaccination on unfavorable clinical outcomes including hospitalization, severe COVID-19, intensive care unit (ICU) admission, and death using a real-world model.
-
Methods
- This single-center retrospective cohort study involved patients with COVID-19 aged ≥18 years who presented to the COVID-19 emergency room at a secondary referral teaching hospital between June 1, 2021 and August 31, 2021. We used a binary logistic regression model to assess the effect of COVID-19 vaccination on unfavorable clinical outcomes, with age, sex, and comorbidities as confounding variables.
-
Results
- A total of 716 patients were included, 32.1% of whom were vaccinated. The elderly participants (≥65 years) had the lowest vaccine coverage among age groups. Vaccination had an effectiveness of 50% (95% confidence interval [CI], 25 to 66) for preventing hospitalization, 97% (95% CI, 77 to 99) for preventing severe COVID-19, 95% (95% CI, 56 to 99) for preventing ICU admission, and 90% (95% CI, 22 to 99) for preventing death. Interestingly, patients with type 2 diabetes had a 2-fold to 4-fold elevated risk of unfavorable outcomes.
-
Conclusions
- Among adults, COVID-19 vaccination has a moderate preventive impact on hospitalization but a high preventive impact on severe COVID-19, ICU admission, and death. The authors suggest that relevant parties increase COVID-19 vaccination coverage, especially in the elderly population.
- Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which spread rapidly and became a global pandemic with a catastrophic worldwide impact on health, economies, and society [1]. Between identification of the first case (on March 6, 2020) and February 2, 2023, COVID-19 infected 6.7 million people and caused 170 000 deaths in Indonesia [2]. As an RNA virus, SARS-CoV-2 has a higher mutation rate than DNA viruses; consequently, new variants have appeared, including the Delta variant [3]. The Delta variant was first detected on October 5, 2020 in India, and Southeast Asia became a hotspot—with Indonesia as the epicenter—between June 2021 and August 2021 [4]. The Indonesian government began a COVID-19 vaccination program in January 2021. The Delta variant became the dominant variant in several other Southeast Asian countries, such as Brunei, Malaysia, Myanmar, Singapore, and Vietnam [5]. The Delta variant is highly transmissible in indoor settings such as households, and it dominated the fourth wave of cases that occurred in Korea around October 2021 [6]. A study reported that the Delta variant had a lower risk of severe clinical outcomes than the Alpha variant [7]. However, this should be interpret in light of findings that the Delta variant may cause more severe disease profiles than the Alpha variant, but improvements in patient management and vaccination could protect patients with COVID-19 from worsened disease progression [8]. In Indonesia, the highest number of deaths occurred during the period when the Delta variant dominated [2].
- Two years after COVID-19 was declared a global pandemic by the World Health Organization, several vaccine candidates were granted emergency use authorization in Indonesia, of which CoronaVac (manufactured by Sinovac) was the first vaccine used. In the vaccination program in Indonesia, healthcare workers were prioritized first. The elderly and public sector workers were the second priority, after which vaccination was extended to members of the public aged ≥18 years [9]. By a few months later, other COVID-19 vaccines, such as ChAdOx1 (manufactured by Oxford–AstraZeneca), mRNA-1273 (Moderna), NVX-CoV2373 (Novavax), BNT162b2 mRNA (Pfizer–BioNTech), and several others were also being used in Indonesia. In clinical trials, several vaccines have been shown to have favorable safety and efficacy, and new vaccines have also been tested for novel SARS-CoV-2 variants [10-14].
- COVID-19 infections in vaccinated people (breakthrough infections) have been recorded [15]. Ideally, through the immunological response elicited by the vaccine, unfavorable clinical outcomes can be prevented or reduced relative to unvaccinated patients [16]. However, several previous studies have revealed that the clinical outcomes of patients with confirmed COVID-19 are influenced by various determinants, including epidemiological and clinical factors; this research indicates that age and comorbidities are the main determinants of clinical outcomes in COVID-19 cases [17,18].
- Breakthrough infections have been examined in Indonesia. A test-negative case-control study conducted in Bali, Indonesia revealed that CoronaVac was 71.1% effective in preventing hospitalization and 87.4% effective in preventing death in participants at least 18 years of age [19]. However, research involving real-world models of COVID-19 vaccine effectiveness in Indonesia is still limited. Therefore, this study was conducted to assess the impact of COVID-19 vaccination on hospitalization, severe COVID-19, intensive care unit (ICU) admission, and death with a real-world model at a COVID-19 referral hospital in West Sumatra during the period when the Delta variant predominated.
INTRODUCTION
- Patients
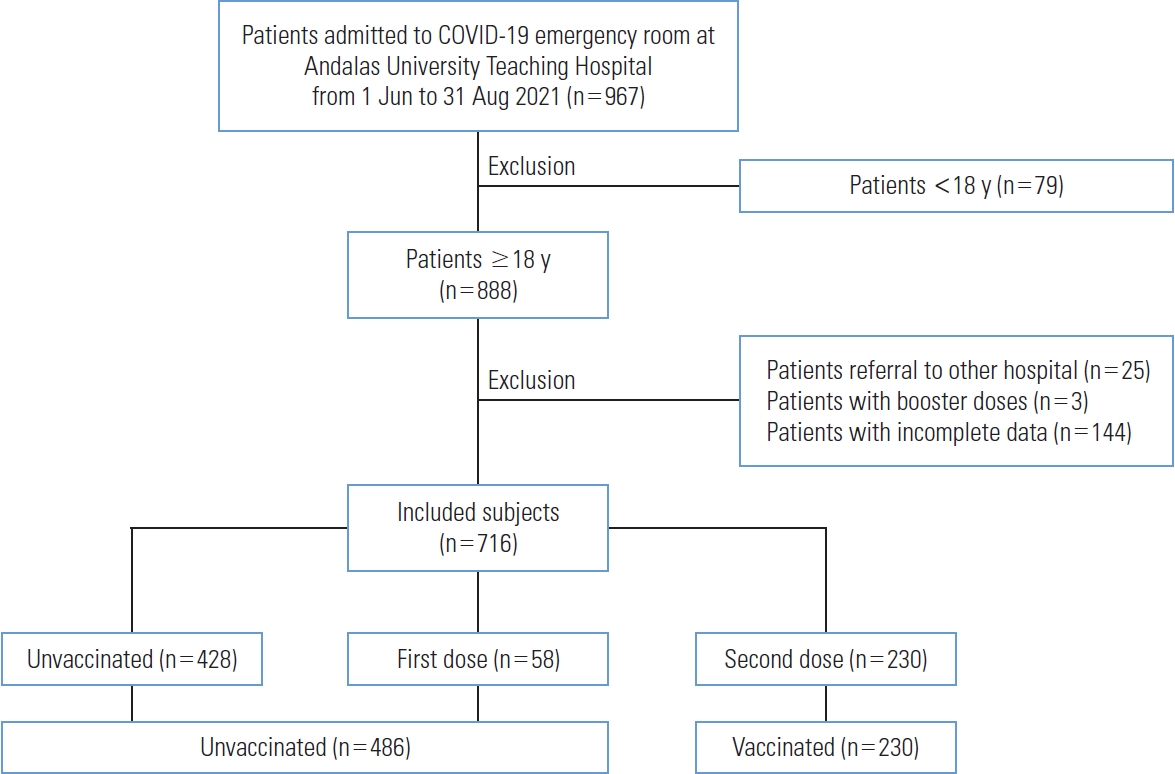
- This single-center retrospective cohort study involved patients with COVID-19 during the second wave of COVID-19 cases in Indonesia, during which the Delta variant predominated [4,20]. The participants were patients who presented to the COVID-19 emergency room at Andalas University Teaching Hospital between June 1, 2021 and August 31, 2021. These patients visited Andalas University Teaching Hospital for several reasons, including positive real-time polymerase chain reaction (RT-PCR) results from other hospitals, public health centers, or elsewhere; symptoms related to COVID-19; a history of contact with COVID-19 patients; and a history of out-of-town travel. A doctor then clinically assessed the patients to determine whether they required hospitalization or ambulatory care. The inclusion criteria for this study were confirmation of COVID-19 by RT-PCR results and an age of at least 18 years. Andalas University Teaching Hospital is a secondary referral hospital equipped for the treatment of patients with mild to severe COVID-19. Critical cases requiring advanced treatment were referred to a tertiary hospital and excluded from this study. The study flowchart is shown in Figure 1.
- Baseline Data Collection
- We collected data on age, sex, comorbidities, and symptoms on admission. Comorbidities were obtained from medical records based on a physical examination, laboratory workup, and/or other relevant information. We used the United States Social Security Administration criterion for old age (≥65 years). For analysis, we separated hypertension and stroke from cardiovascular disease to avoid multicollinearity. Oxygen therapy is a primary treatment for COVID-19. Patients were administered oxygen therapy when their peripheral oxygen saturation (SpO2) was <93% in room air by employing a nasal cannula, a non-rebreathing mask, or a high-flow nasal cannula; a mechanical ventilator was considered in cases of increasing hypoxia, desaturation, or clinical worsening [21]. We collected data regarding the maximum oxygen supplementation therapy administered at the hospital from patient medical records.
- Vaccination Status
- COVID-19 vaccination status was tracked using the Indonesian governmental COVID-19 vaccination application (P-Care BPJS, https://pcare.bpjs-kesehatan.go.id/vaksin/Login) by inputting patients’ national identification numbers. Participants were categorized by vaccination status as unvaccinated (received no dose, received only first dose, or received second dose <14 days after the first dose) or vaccinated (had a positive RT-PCR result for at least 14 days after injection of the second dose). This study excluded patients for whom data were incomplete; hence, the search was imprecise.
- Clinical Outcomes
- We analyzed unfavorable clinical outcomes of patients, including hospitalization, severe COVID-19, ICU admission, and mortality. The Indonesian Society of Pulmonology, the Indonesian Cardiovascular Association, the Indonesian Society of Internal Medicine, the Indonesian Society of Anesthesiology and Intensive Therapy, and the Indonesian Pediatric Society have developed COVID-19 guidelines [21], which were followed to assess the severity of COVID-19 among patients in this study. COVID-19 severity was assessed based on symptoms, the presence of pneumonia, and respiratory distress, then grouped into 5 categories (asymptomatic, mild, moderate, severe, and critical). Severe COVID-19 was defined based on clinical signs of pneumonia (fever, cough, shortness of breath, and rapid breathing) along with 1 of the following conditions: respiratory rate >30 breath/min, severe respiratory distress, or SpO2 <93% in room air in the hospital. Ideally, patients with asymptomatic or mild infection are voluntarily quarantined at home or in quarantine facilities for a maximum of 10 days from the appearance of symptoms, plus 3 days symptom-free. Patients with moderate to severe disease were treated at Andalas University Teaching Hospital, a COVID-19 referral hospital. Hospitalization related to COVID-19 was defined based on RT-PCR confirmation followed by hospitalization at Andalas University Teaching Hospital. COVID-19-related ICU admission was considered to apply to RT-PCR-confirmed cases requiring intensive care at Andalas University Teaching Hospital. COVID-19-related death was defined as all-cause death with a positive RT-PCR result. All data were retrospectively obtained from medical records.
- Statistical Analysis
- Data were analyzed statistically in univariate, bivariate, and multivariable analyses. Univariate analyses were performed to examine the frequencies of age, sex, comorbidities, initial symptom, vaccination status, type of vaccine received, and unfavorable clinical outcomes. Continuous variables were presented as mean±standard deviation if distributed normally or as median (interquartile range) if distributed non-normally. Bivariate analysis was performed to examine the association between independent variables such as age, sex, comorbidities, initial symptom, and unfavorable clinical outcomes, and the dependent variable was vaccination status. The chi-square test (or the Fisher exact test, when 1 or more expected cell counts in the cross-tabulation were less than 5) was performed for categorical variables in the baseline data. The Mann-Whitney U test (or the Kruskal-Wallis test when the data did not meet the normality assumption) was performed for numerical variables in the baseline data. A p-value of less than 0.05 was considered to indicate statistical significance. Multivariable binary logistic regression was performed to identify the factors associated with unfavorable clinical outcomes, namely hospitalization, severe COVID-19, ICU admission, and mortality related to COVID-19. First, the associations between explanatory variables and clinical outcomes were analyzed separately; then, crude odds ratios (ORs) with 95% confidence intervals (CIs) were obtained. Second, multivariable analysis was performed on variables for which p-value <0.1, and adjusted odds ratios (aORs) with 95% CIs were obtained. Variables with p-value<0.05 in the second step were considered factors associated with clinical outcomes due to the statistical significance. Before the multivariable binary logistic regression, we performed collinearity diagnostics to avoid multicollinearity, particularly for the variables of hypertension, stroke, and cardiovascular disease. The variance inflation factor (VIF) technique was used, with a VIF score of >10 considered to indicate that the variables were very strongly correlated. The Hosmer-Lemeshow test was used to determine the goodness of the multivariable analysis model. We excluded variables when their inclusion did not result in model goodness-of-fit (p<0.05). To calculate the vaccine effectiveness, we used the following formula: vaccine effectiveness=(1−aOR)×100. All statistical analyses were performed with SPSS version 25 (IBM Corp., Armonk, NY, USA).
- Ethics Statement
- The Ethics Commission of the Andalas University Faculty of Medicine provided ethical clearance for this study, with approval number 798/UN.16.2/KEP-FK/2022. The requirement for informed consent was waived due to the retrospective nature of the research, and the data were processed anonymously.
METHODS
- Demographic Characteristics, Clinical Characteristics, and Outcomes of Patients Based on Vaccination Status
- From June 1, 2021 to August 31, 2021, 716 patients with COVID-19 were included in this study. The details of patient inclusion are shown in Figure 1. Demographic information based on patient vaccination status is shown in Table 1. Among the patients, 67.9% were unvaccinated. This unvaccinated group consisted of 428 patients who had received no COVID-19 vaccination and 58 patients who had received the first dose. Regarding age, most patients were non-elderly, and the elderly group was predominantly unvaccinated (a significant finding). Most vaccinated patients had no comorbidity. The unvaccinated participants had significantly more comorbidities than the vaccinated patients, including type 2 diabetes, stroke, and renal disease. Initial symptoms of dyspnea and cough were significantly more common among the unvaccinated participants, whereas hyposmia and hypogeusia were significantly more frequent in the vaccinated patients. It should be noted, however, that the symptoms were subjective and largely depended on patient reports. Required oxygen supplementation with either a non-rebreathing mask or a high-flow nasal cannula was significantly more common in the unvaccinated group than among vaccinated patients. Nasal cannula and mechanical ventilation use were more common in the unvaccinated group, but this finding was not statistically significant. Additionally, the unvaccinated patients had significantly higher incidence rates of COVID-19-related hospitalization (44.8 vs. 22.7%; p<0.001), severe COVID-19 (15.4 vs. 0.4%; p<0.001), ICU admission (8.8 vs. 0.4%; p<0.001), and death (6.8 vs. 0.4%; p<0.001).
- Type of Vaccine
- The vaccination program in Indonesia started in February 2021, and CoronaVac (Sinovac) was the first vaccine used. Therefore, CoronaVac was used in the most patients, followed by ChAdOx1 (Oxford–AstraZeneca), mRNA-1273 (Moderna), and BNT162b2 mRNA (Pfizer–BioNTech). The manufacturer and type of each vaccine are detailed in Table 2.
- Effect of Vaccination on COVID-19-related Hospitalization
- Vaccination as a public health intervention is highly effective in preventing, controlling, and modifying unfavorable clinical outcomes of COVID-19 [22]. To investigate whether vaccination was associated with COVID-19-related hospitalization in this study, multivariable binary logistic regression was performed with age, sex, and comorbidities as confounding variables. VIF values <10 were found for all variables, indicating the lack of very strong statistical correlations between variables. In the unadjusted and adjusted analyses, old age (OR, 2.52; 95% CI, 1.43 to 4.45; p=0.001), at least 1 comorbidity (OR, 5.67; 95% CI, 3.73 to 8.62; p<0.001), and type 2 diabetes (OR, 2.17; 95% CI, 1.13 to 4.14; p=0.020) were associated with increased incidence of hospitalization. In contrast, vaccination (OR, 0.50; 95% CI, 0.34 to 0.75; p=0.001) was associated with a lower incidence of hospitalization. We excluded stroke, liver disease, renal disease, and cardiovascular disease as factors of hospitalization because a p-value <0.05 was found on the Hosmer-Lemeshow test for the model including these variables. The details are shown in Table 3.
- Effect of Vaccination on Severe COVID-19
- To understand the risk factors for severe COVID-19, multivariable binary logistic regression was used. The results showed that old age (OR, 2.86; 95% CI, 1.41 to 5.81; p=0.004), at least 1 comorbidity (OR, 3.49; 95% CI, 1.41 to 8.63; p=0.007), type 2 diabetes (OR, 4.44; 95% CI, 2.17 to 9.08; p<0.001), respiratory disease (OR, 3.79; 95% CI, 1.168 to 8.58; p=0.001), and renal disease (OR, 9.58; 95% CI, 2.09 to 43.90; p=0.004) were associated with a higher incidence of severe infection. Vaccination (OR, 0.03; 95% CI, 0.01 to 0.23; p=0.001) was associated with a lower incidence of severe COVID-19. The details are shown in Table 3.
- Effect of Vaccination on COVID-19-related Intensive Care Unit Admission
- To understand the risk factors for COVID-19-related ICU admission, multivariable binary logistic regression was used. The results showed that hypertension (OR, 2.76; 95% CI, 1.18 to 6.47; p=0.020), type 2 diabetes (OR, 4.02; 95% CI, 1.79 to 9.03; p=0.001), respiratory disease (OR, 7.16; 95% CI, 2.98 to 17.21; p<0.001), liver disease (OR, 5.69; 95% CI, 1.81 to 17.84; p=0.030), and renal disease (OR, 6.32; 95% CI, 1.72 to 23.26; p=0.007) were associated with a higher incidence of ICU admission, and vaccination (OR, 0.05; 95% CI, 0.01 to 0.44; p=0.003) was associated with a lower incidence. The details are shown in Table 4.
- Effect of Vaccination on COVID-19-related Death
- To understand the risk factors for COVID-19-related death, multivariable binary logistic regression was performed. The result showed that old age (OR, 4.60; 95% CI, 2.02 to 10.51; p<0.001), at least 1 comorbidity (OR, 7.25; 95% CI, 1.94 to 27.08; p=0.003), and diabetes type 2 (OR, 3.15; 95% CI, 1.38 to 7.21; p=0.007) were associated with increased incidence of death, while vaccination (OR, 0.10; 95% CI, 0.01 to 0.78; p=0.028) was associated with decreased incidence. We excluded renal disease because a p-value <0.05 was found in the Hosmer-Lemeshow test for the model including this variable. The details are shown in Table 4.
- Interesting results were observed regarding type 2 diabetes and vaccination. These factors predicted all of the unfavorable clinical outcomes with statistical significance. Patients with type 2 diabetes had a 2- fold to 4-fold higher risk of unfavorable outcomes. Vaccination provided 50% protection against hospitalization, 97% against severe COVID-19, 95% against ICU admission, and 90% against death in patients with confirmed COVID-19.
RESULTS
- COVID-19 vaccine development was a scientific success in controlling the SARS-CoV-2 pandemic in the population [23]. However, several major waves of cases have been recorded since a vaccination program was initiated in Indonesia. In this study, we assessed the effectiveness of the COVID-19 vaccine on clinical outcomes by considering public health determinants such as age, sex, and comorbidities in a real-world model during a period when the Delta variant predominated. Our research yielded interesting results. However, certain points must be carefully interpreted.
- First, within the first 5 months of implementing the COVID19 vaccination program in Indonesia, we found that only 32.1% of patients in West Sumatra were vaccinated, and the elderly participants had the lowest coverage among age groups. This finding indicates a mismatch between Indonesian government policies and the societal reality. The vaccination program in Indonesia began in January 2021, with healthcare workers prioritized. The elderly population was the second priority for vaccination along with groups of public sector workers; afterward, vaccination was extended to members of the public aged ≥18 years [9,24]. In Indonesia, prior to the administration of the COVID-19 vaccine to the elderly (those >60 years old), vaccination eligibility was determined based on frailty (ICD code R54); this was assessed using the RAPUH questionnaire, a validated tool adapted from the FRAIL questionnaire [25]. Elderly people with a frailty score above 2 points were recommended against receiving the vaccine [26]. Most of the elderly population in the present study had comorbidities. We were unable to assess whether the elderly patients in the sample did not meet the vaccination requirements or exhibited vaccine hesitancy. The low vaccine coverage rate in this study may be due to vaccine hesitancy, which is relatively common in Indonesia and also exists elsewhere [27,28]. One study with participants from 7 provinces in Indonesia conducted prior to the vaccination program indicated high acceptance of a COVID-19 vaccine, assuming 95% effectiveness [29]. Currently, education, age, comorbidities, and distrust of government policy are vaccine hesitancy factors in Indonesia [28,30]. West Sumatra is an Indonesian province with a strong adherence to Islamic culture. Lack of belief that a vaccine is halal may underlie public reluctance to receive vaccination [30]. In addition, in the adjustment analysis of this study, old age was associated with increased incidence of hospitalization, severe COVID-19, ICU admission, and death. Therefore, a specialized strategy is needed from the government to increase COVID-19 vaccination coverage, particularly among the elderly.
- Second, vaccination was associated with a lower incidence of hospitalization, severe COVID-19, ICU admission, and death. Our study confirmed the effectiveness shown by several clinical trials of COVID-19 vaccines in preventing unfavorable outcomes [10-13]. A previous effectiveness study of CoronaVac using a real-world model in Chile from February 2, 2021 to May 1, 2021 in participants over 18 years old found that a full dose of CoronaVac had 65.9% effectiveness against SARS CoV-2 infection, 87.5% effectiveness in preventing hospitalization, 90.3% effectiveness in preventing ICU admission, and 86.3% effectiveness in preventing death [31]. While COVID-19 vaccine effectiveness changes across periods dominated by different variants, vaccination was still highly effective in preventing unfavorable clinical outcomes during the Delta period [32-34]. In developing countries like Indonesia, mathematical modeling estimates indicate that COVID-19 vaccination can prevent as many as 45% of COVID-19-related deaths if vaccination coverage among the population reaches 40% [35].
- The findings of this study regarding the effectiveness of vaccines in preventing COVID-19-related hospitalization must be carefully interpreted. In Indonesia, ideally, patients with mild COVID-19 should voluntarily quarantine at home or in facilities provided by the government. However, patients with asymptomatic to mild disease who lack a place to isolate or who have uncontrolled comorbidities can be hospitalized, including at our institution. Andalas University Teaching Hospital is located on the campus of Andalas University, which serves the public but primarily cared for students, lecturers, and the academic community during the study period. Thus, the 50% effectiveness of COVID-19 vaccination in preventing hospitalization in this study, which is relatively low compared to other studies, may be explained by the reasons above.
- Third, this study identified type 2 diabetes as a risk factor that increased the incidence of all unfavorable clinical outcomes. This finding is noteworthy because type 2 diabetes is a common morbidity worldwide, especially in Indonesia. A 2021 report of the International Diabetes Federation Atlas explained that diabetes affects 536.6 million adults globally, and Indonesia is the country with the fifth greatest number of adults with diabetes (19.5 million) in the world. Indonesia also has the highest proportion of undiagnosed diabetes, at 73.7% (14.3 million people) [36]. A nationwide retrospective cohort study in Korea showed that type 2 diabetes was a predictor of death and fatal adverse outcomes (invasive ventilator use, extracorporeal membrane oxygenation use, multi-organ damage, and death) [37]. A multicenter study in China showed that fasting blood glucose ≥7.0 mmol/L (>126 mg/dL) at admission was an independent predictor of 28-day mortality in patients with COVID-19 without a previous diagnosis of diabetes [38]. A random-effects meta-analysis including 18 studies and 10 studies showed that type 2 diabetes was moderately associated with COVID-19 severity (invasive mechanical ventilation, ICU admission, or SpO2 <90%) and mortality, respectively [39]. Several potential underlying mechanisms have been proposed [40]. Based on this study and others, we recommend peripheral blood glucose screening in patients with newly confirmed COVID-19, either with or without a history of type 2 diabetes.
- Our study had several strengths as well as limitations that should be considered when interpreting the results. Regarding strengths, this was a single-center study with a relatively large amount of data subjected to rigorous inclusion and exclusion criteria. Additionally, this study was conducted at a secondary referral hospital during a large spike in COVID-19 cases in Indonesia, with heterogeneous participant characteristics, severity, and clinical outcomes. However, several limitations of this study are evident. First, the Andalas University Teaching Hospital is a secondary referral hospital that is not designed to treat critical infections involving acute respiratory distress syndrome or sepsis requiring advanced treatment. Critical cases were transferred to tertiary referral hospitals, and we did not include these patients in the analysis because we lacked access to the necessary data. This may have introduced bias in the study results. Second, the number of vaccinated patients with unfavorable clinical outcomes such as severe COVID-19, ICU admission, and death was relatively small. Third, we did not assess clinical factors such as laboratory workup, patient progression, patient treatment, and other confounding factors. Fourth, whole-genome sequencing was not performed in these participants to identify the SARS-CoV-2 variant.
- In conclusion, we found that COVID-19 vaccination has a moderate preventive impact on hospitalization but a high preventive impact on severe COVID-19, ICU admission, and death in the Indonesian population. Accordingly, we suggest that related parties increase the coverage of COVID-19 vaccination, especially in the elderly population.
DISCUSSION
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
Funding was received from the Faculty of Medicine of Universitas Andalas, with grant number 678/ UN16.02.D/PP/2021.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Rahadi DA, Yusri E, Putra SP. Data curation: Rahadi DA. Formal analysis: Rahadi DA, Ilmiawati C. Funding acquisition: Yusri E, Putra SP. Methodology: Rahadi DA, Ilmiawati C. Writing – original draft: Rahadi DA. Writing – review & editing: Yusri E, Putra SP, Semiarty R, Pertiwi D, Ilmiawati C.
Notes
ACKNOWLEDGEMENTS
| Characteristics | Unvaccinated (n=487) | Vaccinated (n=229) | p-value1 | |
|---|---|---|---|---|
| Age, median [IQR] (y) | 34 [30] | 46 [38] | <0.001 | |
| Elderly, ≥65 y | 72 (14.8) | 11 (4.8) | <0.001 | |
| Male | 197 (40.5) | 110 (48.0) | 0.067 | |
| Comorbidities | ||||
| No comorbidity | 250 (51.3) | 164 (71.6) | <0.001 | |
| Hypertension | 82 (16.8) | 27 (11.9) | 0.101 | |
| Diabetes | 54 (11.1) | 12 (5.2) | 0.017 | |
| Stroke | 10 (2.1) | - | 0.035 | |
| Respiratory disease | 40 (8.2) | 11 (4.8) | 0.134 | |
| Liver disease | 22 (4.5) | 14 (6.1) | 0.466 | |
| Renal disease | 13 (2.7) | 1 (0.4) | 0.045 | |
| Cardiovascular disease | 15 (3.1) | 3 (1.3) | 0.204 | |
| Initial symptom | ||||
| Asymptomatic | 32 (6.6) | 15 (6.6) | 1.000 | |
| Fever | 225 (46.2) | 111 (48.5) | 0.626 | |
| History of fever | 89 (18.3) | 35 (15.3) | 0.378 | |
| Dyspnea | 146 (29.9) | 25 (10.9) | <0.001 | |
| Cough | 368 (75.6) | 155 (67.7) | 0.034 | |
| Flu | 85 (17.5) | 54 (23.6) | 0.067 | |
| Sore throat | 25 (5.1) | 18 (7.9) | 0.206 | |
| Hyposmia | 128 (26.3) | 83 (36.2) | 0.008 | |
| Hypogeusia | 58 (11.9) | 12 (5.2) | 0.008 | |
| Nausea | 25 (5.1) | 14 (5.1) | 0.717 | |
| Vomiting | 28 (5.7) | 18 (7.9) | 0.362 | |
| Loss of appetite | 32 (29.2) | 11 (4.8) | 0.447 | |
| Myalgia | 23 (4.7) | 12 (5.2) | 0.910 | |
| Fatigue | 50 (10.3) | 16 (7.0) | 0.202 | |
| Diarrhea | 21 (4.3) | 8 (3.5) | 0.753 | |
| Headache | 50 (10.3) | 28 (12.2) | 0.511 | |
| Oxygen supplementation | ||||
| Nasal cannula | 62 (12.7) | 18 (7.9) | 0.071 | |
| Non-rebreathing mask | 54 (11.1) | 2 (0.9) | <0.001 | |
| High-flow nasal cannula | 11 (2.3) | - | 0.020 | |
| Mechanical ventilation | 4 (0.8) | - | 0.312 | |
| Clinical outcome | ||||
| Hospitalization | 218 (44.8) | 52 (22.7) | <0.001 | |
| Severe COVID-19 | 75 (15.4) | 1 (0.4) | <0.001 | |
| ICU admission | 43 (8.8) | 1 (0.4) | <0.001 | |
| Death | 33 (6.8) | 1 (0.4) | <0.001 | |
| Variables | n (%) |
Hospitalized (n=270) |
n (%) |
Severe (n=76) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | p-value | Adjusted1 | p-value | Unadjusted | p-value | Adjusted1 | p-value | ||||
| Age ≥65 y | 58 (21.5) | 4.61 (2.80, 7.57) | <0.001 | 2.52 (1.43, 4.45) | 0.001 | 31 (40.8) | 7.79 (4.56, 13.34) | <0.001 | 2.86 (1.41, 5.81) | 0.004 | |
| Male | 110 (40.7) | - | 0.369 | - | - | 42 (55.3) | 1.75 (1.08, 2.82) | 0.022 | - | 0.056 | |
| Comorbidities | |||||||||||
| At least 1 comorbidity | 193 (71.5) | 7.75 (5.51, 10.90) | <0.001 | 5.67 (3.73, 8.62) | <0.001 | 68 (89.5) | 14.80 (6.97, 31.22) | <0.001 | 3.49 (1.41, 8.63) | 0.007 | |
| Hypertension | 68 (25.2) | 3.33 (2.18, 5.08) | <0.001 | - | 0.493 | 31 (40.8) | 4.96 (2.96, 8.31) | <0.001 | - | 0.274 | |
| Diabetes | 51 (18.9) | 6.69 (3.68, 12.17) | <0.001 | 2.17 (1.13, 4.14) | 0.020 | 30 (39.5) | 10.90 (6.19, 19.34) | <0.001 | 4.44 (2.17, 9.08) | <0.001 | |
| Stroke | 9 (3.3) | 15.35 (1.93, 121.80) | 0.0012 | - | - | 7 (9.2) | 21.50 (5.45, 85.21) | <0.001 | - | 0.055 | |
| Respiratory disease | 34 (12.6) | 3.64 (1.99, 6.65) | <0.001 | - | 0.338 | 18 (23.7) | 5.71 (3.03, 10.77) | <0.001 | 3.79 (1.68, 8.58) | 0.001 | |
| Liver disease | 30 (11.1) | 9.17 (3.76, 22.33) | <0.0012 | - | - | 10 (13.2) | 3.58 (1.65, 7.75) | 0.001 | - | 0.053 | |
| Renal disease | 13 (4.8) | 22.51 (2.93, 173.07) | 0.0032 | - | - | 10 (13.2) | 24.10 (7.35, 78.94) | 0.001 | 9.58 (2.09, 43.90) | 0.004 | |
| Cardiovascular disease | 14 (5.2) | 6.04 (1.97, 18.55) | <0.0012 | - | - | 7 (9.2) | 5.80 (2.19, 15.45) | <0.001 | - | 0.460 | |
| Vaccinated | 54 (20.0) | 0.36 (0.25, 0.52) | <0.001 | 0.50 (0.34, 0.75) | 0.001 | 1 (1.3) | 0.02 (0.01, 0.17) | <0.001 | 0.03 (0.01, 0.23) | 0.001 | |
| Variables | n (%) |
Admitted to ICU (n=44) |
n (%) |
Death (n=34) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | p-value | Adjusted1 | p-value | Unadjusted | p-value | Adjusted1 | p-value | ||||
| Age ≥65 y | 14 (31.8) | 4.08 (2.06, 8.06) | <0.001 | - | 0.970 | 17 (50.0) | 9.33 (4.55, 19.15) | <0.001 | 4.60 (2.02, 10.51) | <0.001 | |
| Male | 24 (54.5) | - | 0.109 | - | 20 (58.8) | 1.97 (0.98, 3.96) | 0.058 | - | 0.053 | ||
| Comorbidities | |||||||||||
| At least 1 comorbidity | 40 (90.9) | 15.65 (5.53, 44.25) | <0.001 | - | 0.102 | 3 (8.8) | 15.70 (4.74, 51.77) | <0.001 | 7.25 (1.94, 27.08) | 0.003 | |
| Hypertension | 20 (45.5) | 5.46 (2.90, 10.29) | <0.001 | 2.76 (1.18, 6.47) | 0.020 | 10 (29.4) | 2.45 (1.14, 5.29) | 0.022 | - | 0.139 | |
| Diabetes | 19 (43.2) | 10.11 (5.19, 19.67) | <0.001 | 4.02 (1.79, 9.03) | 0.001 | 14 (41.2) | 8.48 (4.05, 17.76) | <0.001 | 3.15 (1.38, 7.21) | 0.007 | |
| Stroke | 4 (9.1) | 11.10 (3.01, 40.92) | <0.001 | - | 0.196 | 2 (5.9) | 5.27 (1.07, 25.81) | 0.041 | - | 0.801 | |
| Respiratory disease | 15 (34.1) | 9.14 (4.50, 18.55) | <0.001 | 7.16 (2.98, 17.21) | <0.001 | 7 (20.6) | 3.76 (1.55, 9.12) | 0.003 | - | 0.297 | |
| Liver disease | 7 (15.9) | 4.20 (1.72, 10.21) | 0.002 | 5.69 (1.81, 17.84) | 0.030 | 3 (8.8) | - | 0.307 | - | - | |
| Renal disease | 7 (15.9) | 17.97 (5.99, 53.92) | <0.001 | 6.32 (1.72, 23.26) | 0.007 | 7 (20.6) | 25.00 (8.19, 76.33) | <0.0012 | - | - | |
| Cardiovascular disease | 1 (2.3) | - | 0.916 | - | 4 (11.8) | 6.36 (1.98, 20.50) | 0.002 | - | 0.411 | ||
| Vaccinated | 1 (2.3) | 0.31 (0.01, 0.23) | 0.001 | 0.05 (0.01, 0.44) | 0.003 | 1 (2.9) | 0.04 (0.01, 0.31) | 0.002 | 0.10 (0.01, 0.78) | 0.028 | |
Values are presented as odds ratio (95% confidence interval).
COVID-19, coronavirus disease 2019; ICU, intensive care unit.
1 Calculated by excluding variables for which p≥0.1.
2 Excluded in the adjusted model because the p-value of the Hosmer-Lemeshow test was <0.05 with the inclusion of these variables.
- 1. Clemente-Suárez VJ, Navarro-Jiménez E, Moreno-Luna L, Saavedra-Serrano MC, Jimenez M, Simón JA, et al. The impact of the COVID-19 pandemic on social, health, and economy. Sustainability 2021;13(11):6314Article
- 2. Satgas COVID-19. Daily progress of positive confirmed cases of COVID-19 in Indonesia. 2023 [cited 2023 Feb 2]. Available from: https://covid19.go.id/peta-sebaran (Indonesian)
- 3. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 2021;19(3):155-170ArticlePubMedPMCPDF
- 4. Dyer O. Covid-19: Indonesia becomes Asia’s new pandemic epicentre as delta variant spreads. BMJ 2021;374: n1815ArticlePubMed
- 5. Chu DT, Vu Ngoc SM, Vu Thi H, Nguyen Thi YV, Ho TT, Hoang VT, et al. COVID-19 in Southeast Asia: current status and perspectives. Bioengineered 2022;13(2):3797-3809ArticlePubMedPMC
- 6. Hwang H, Lim JS, Song SA, Achangwa C, Sim W, Kim G, et al. Transmission dynamics of the delta variant of SARS-CoV-2 infections in South Korea. J Infect Dis 2022;225(5):793-799ArticlePubMedPDF
- 7. Florensa D, Mateo J, Spaimoc R, Miret C, Godoy S, Solsona F, et al. Severity of COVID-19 cases in the months of predominance of the Alpha and Delta variants. Sci Rep 2022;12(1):15456ArticlePubMedPMCPDF
- 8. Hu Z, Huang X, Zhang J, Fu S, Ding D, Tao Z. Differences in clinical characteristics between delta variant and wild-type SARS-CoV-2 infected patients. Front Med (Lausanne) 2022;8: 792135ArticlePubMedPMC
- 9. Ministry of Health of the Republic of Indonesia. The implementation of vaccination in the context of combating corona virus disease 2019 pandemic (COVID-19). 2021 [cited 2023 Feb 1]. Available from: https://peraturan.bpk.go.id/Home/Details/169665/permenkes-no-10-tahun-2021 (Indonesian)
- 10. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARSCoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021;398(10296):213-222PubMedPMC
- 11. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med 2021;384(23):2187-2201ArticlePubMedPMC
- 12. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383(27):2603-2615ArticlePubMedPMC
- 13. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 2021;385(13):1172-1183ArticlePubMedPMC
- 14. Viveiros-Rosa SG, Mendes CD, Farfán-Cano GG, El-Shazly M. The race for clinical trials on Omicron-based COVID-19 vaccine candidates: updates from global databases. Narra J 2022;2(3):e88ArticlePubMedPMCPDF
- 15. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, Elbaz M, Nesher L, Stein M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID19 patients in Israel. Clin Microbiol Infect 2021;27(11):1652-1657ArticlePubMedPMC
- 16. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol 2021;21(8):475-484ArticlePubMedPMCPDF
- 17. Henkens MT, Raafs AG, Verdonschot JA, Linschoten M, van Smeden M, Wang P, et al. Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study. BMC Geriatr 2022;22(1):184ArticlePubMedPMC
- 18. Izcovich A, Ragusa MA, Tortosa F, Lavena Marzio MA, Agnoletti C, Bengolea A, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One 2020;15(11):e0241955ArticlePubMedPMC
- 19. Suryatma A, Anasi R, Hananto M, Hermawan A, Ramadhany R, Indalao IL, et al. Effectiveness of the inactivated COVID-19 vaccine (CoronaVac) in adult population in Bali, Indonesia. medRxiv [Preprint]; 2022 [cited 2023 Feb 2]. Available from: https://doi.org/10.1101/2022.02.02.22270351Article
- 20. Cahyani I, Putro EW, Ridwanuloh AM, Wibowo S, Hariyatun H, Syahputra G, et al. Genome profiling of SARS-CoV-2 in Indonesia, ASEAN and the neighbouring East Asian countries: features, challenges and achievements. Viruses 2022;14(4):778ArticlePubMedPMC
- 21. Burhan E, Dwi Susanto A, Isbaniah F, Aman Nasution S, Ginanjar E, Wicaksono Pitoyo C, et al. 4th COVID-19 guidelines of the Indonesian Society of Pulmonology (PDPI), the Indonesian Cardiovascular Association (PERKI), the Indonesian Society of Internal Medicine (PAPDI), the Indonesian Society of Anesthesiology and Intensive Therapy (PERDATIN), and the Indonesian Pediatric Society (IDAI). 2022 [cited 2023 Feb 1]. Available from: https://covid19.go.id/storage/app/media/Protokol/2022/Februari/Buku%20Tatalaksana%20COVID-19%205%20OP%20Edisi%204%20Jan%202022.pdf (Indonesian)
- 22. Patel MD, Rosenstrom E, Ivy JS, Mayorga ME, Keskinocak P, Boyce RM, et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortality. JAMA Netw Open 2021;4(6):e2110782ArticlePubMedPMC
- 23. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med 2020;382(21):1969-1973ArticlePubMed
- 24. Nugraha RR, Miranda AV, Ahmadi A, Lucero-Prisno DE 3rd. Accelerating Indonesian COVID-19 vaccination rollout: a critical task amid the second wave. Trop Med Health 2021;49(1):76ArticlePubMedPMCPDF
- 25. Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012;16(7):601-608ArticlePubMedPMCPDF
- 26. Indonesia Society of Internal Medicine. PAPDI recommendations on the administration of COVID-19 vaccination in patients with comorbidities; 2021 Mar 18 [cited 2023 Feb 3]. Available from: https://www.papdi.or.id/%20berita/info-papdi/1024-rekomendasi-papdi-tentang-pemberian-vaksinasi-covid-19-pada-pasien-dengan-penyakit-penyerta-komorbid-revisi-18-maret-2021 (Indonesian)
- 27. Rosiello DF, Anwar S, Yufika A, Adam RY, Ismaeil MI, Ismail AY, et al. Acceptance of COVID-19 vaccination at different hypothetical efficacy and safety levels in ten countries in Asia, Africa, and South America. Narra J 2021;1(3):e55ArticlePubMedPMCPDF
- 28. Sugiri AH, Yenny Y. Determinants of COVID-19 vaccine hesitancy: a community-based study in Dumai City, Riau Province, Indonesia. Open Access Maced J Med Sci 2022;10(E):1420-1425ArticlePDF
- 29. Harapan H, Wagner AL, Yufika A, Winardi W, Anwar S, Gan AK, et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Front Public Health 2020;8: 381ArticlePubMedPMC
- 30. Utami A, Margawati A, Pramono D, Nugraheni A, Pramudo SG. Determinant factors of COVID-19 vaccine hesitancy among adult and elderly population in Central Java, Indonesia. Patient Prefer Adherence 2022;16: 1559-1570ArticlePubMedPMCPDF
- 31. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385(10):875-884ArticlePubMedPMC
- 32. Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative casecontrol real-world study. Emerg Microbes Infect 2021;10(1):1751-1759ArticlePubMedPMC
- 33. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Aghayari Sheikh Neshin S, Khatami A, et al. Effectiveness of COVID-19 vaccines against Delta (B.1.617.2) variant: a systematic review and meta-analysis of clinical studies. Vaccines (Basel) 2021;10(1):23ArticlePubMedPMC
- 34. Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med 2021;27(12):2136-2143ArticlePubMedPDF
- 35. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022;22(9):1293-1302ArticlePubMedPMC
- 36. International Diabetes Federation (IDF). IDF diabetes atlas 10th edition. 2021 [cited 2023 Feb 1]. Available from: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf
- 37. Song J, Park DW, Cha JH, Seok H, Kim JY, Park J, et al. Clinical course and risk factors of fatal adverse outcomes in COVID-19 patients in Korea: a nationwide retrospective cohort study. Sci Rep 2021;11(1):10066ArticlePubMedPMCPDF
- 38. Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 2020;63(10):2102-2111ArticlePubMedPMCPDF
- 39. de Almeida-Pititto B, Dualib PM, Zajdenverg L, Dantas JR, de Souza FD, Rodacki M, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr 2020;12: 75ArticlePubMedPMCPDF
- 40. Unnikrishnan R, Misra A. Diabetes and COVID19: a bidirectional relationship. Nutr Diabetes 2021;11(1):21ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Changes in the intrinsic severity of severe acute respiratory syndrome coronavirus 2 according to the emerging variant: a nationwide study from February 2020 to June 2022, including comparison with vaccinated populations
Boyeong Ryu, Eunjeong Shin, Dong Hwi Kim, HyunJu Lee, So Young Choi, Seong-Sun Kim, Il-Hwan Kim, Eun-Jin Kim, Sangwon Lee, Jaehyun Jeon, Donghyok Kwon, Sungil Cho
BMC Infectious Diseases.2024;[Epub] CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite