Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 55(4); 2022 > Article
-
Systematic Review
Vitamin D Deficiency and Comorbidities as Risk Factors of COVID-19 Infection: A Systematic Review and Meta-analysis -
Pinki Mishra1
 , Rizwana Parveen1
, Rizwana Parveen1 , Ram Bajpai2
, Ram Bajpai2 , Nidhi Agarwal1
, Nidhi Agarwal1
-
Journal of Preventive Medicine and Public Health 2022;55(4):321-333.
DOI: https://doi.org/10.3961/jpmph.21.640
Published online: June 13, 2022
1Centre for Translational and Clinical Research, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi, India
2School of Medicine, Keele University, Staffordshire, UK
- Corresponding author: Nidhi Agarwal, Centre for Translational and Clinical Research, School of Chemical and Life Sciences, Jamia Hamdard, New Delhi 110062, India, E-mail: nidhi.bharal@gmail.com
Copyright © 2022 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Extensive evidence links low vitamin D status and comorbidities with coronavirus disease 2019 (COVID-19) outcomes, but the results of published studies are contradictory. Therefore, we investigated the association of lower levels of vitamin D and comorbidities with the risk of COVID-19 infection.
-
Methods
- We searched MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for articles published until August 20, 2021. Sixteen eligible studies were identified (386 631 patients, of whom 181 114 were male). We included observational cohort and case-control studies that evaluated serum levels of vitamin D in COVID-19-positive and COVID-19-negative patients. Mean differences (MDs) with 95% confidence intervals (CIs) were calculated.
-
Results
- Significantly lower vitamin D levels were found in COVID-19-positive patients (MD, −1.70; 95% CI, −2.74 to −0.66; p=0.001), but with variation by study design (case-control: −4.04; 95% CI, −5.98 to −2.10; p<0.001; cohort: −0.39; 95% CI, −1.62 to 0.84; p=0.538). This relationship was more prominent in female patients (MD, −2.18; 95% CI, −4.08 to −0.28; p=0.024) than in male patients (MD, −1.74; 95% CI, −3.79 to 0.31; p=0.096). Male patients showed higher odds of having low vitamin D levels (odds ratio [OR], 2.09; 95% CI, 1.38 to 3.17; p<0.001) than female patients (OR, 1.17; 95% CI, 0.74 to 1.86; p=0.477). Comorbidities showed inconsistent, but generally non-significant, associations with COVID-19 infection.
-
Conclusions
- Low serum vitamin-D levels were significantly associated with the risk of COVID-19 infection. This relationship was stronger in female than in male COVID-19 patients. Limited evidence was found for the relationships between comorbidities and COVID-19 infection, warranting large population-based studies to clarify these associations.
- The world is in the grasp of the coronavirus disease 2019 (COVID-19) pandemic, which began in late 2019 in Wuhan, China. The infectious agent responsible for COVID-19 was originally called 2019-nCoV; the disease was renamed COVID-19, and its causative agent was renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), by the World Health Organization on February 11, 2020. Previous coronavirus epidemics included that of severe acute respiratory syndrome SARS-CoV, initiated in China in 2002, and that of Middle East respiratory syndrome (MERS)-CoV, first recorded in 2012. These epidemics all started with the spread of the infection between animals and humans. The primary cause of death is usually due to extreme atypical pneumonia [1]. To date, there is no established curative therapy for this virus, and prevention remains the best strategy for combating the COVID-19 pandemic.
- Vitamin D supplementation is considered to be a preventive strategy, as indicated by certain observational studies [2–4]. Insufficiency of vitamin D is a public health problem affecting over a billion people across all life stages worldwide [5]. In the past decade, several studies have demonstrated a potential link between vitamin D deficiency and various diseases, including systemic infections [6–8]. Various clinical studies have reported associations of low serum vitamin D levels with acute respiratory tract infections, including epidemic influenza [9–11]. Vitamin D, a steroid hormone, whose biosynthesis begins with solar ultraviolet radiation in bare skin exposed to strong sunlight, and shows multidimensional effects beyond calcium and bone metabolism. Vitamin D improves mucosal defenses by secreting antiviral peptides [12,13]. Vitamin D receptors are highly expressed in B and T lymphocytes, suggesting that they may play a role in modulating the innate and adaptive immune responses [14].
- Vitamin D levels can be affected by many factors such as sun exposure, genetics, supplementation, and comorbidities. Vitamin D levels decrease during winter, and low vitamin D levels are associated with an increased risk of acute respiratory tract infections during winter [15] mitigated by vitamin D supplementation. Several well-defined conditions have been recognized as risk factors for a worsening disease course and poor COVID-19 outcomes. The most important risk factors that may result in severe COVID-19 symptomatology are diabetes mellitus, hypertension, age over 65 years, obesity, and immunosuppressive therapy. By diverse mechanisms, these circumstances are hypothesized to alter host responses to infection, enhancing and ramping up harmful pathophysiological processes. Initial viral immune evasion and the ensuing hyperinflammatory response resulting from excessive and undirected immune activation are crucial components in COVID-19 pathogenesis [16,17]. People older than 60 years of age with hypertension, diabetes, and respiratory, cardiovascular, cerebrovascular, liver, kidney and gastrointestinal disorders are more vulnerable to COVID-19 infection and experience higher mortality. Because of the limited number of patients, the involvement of malignant conditions is under debate [18].
- Extensive evidence has recently linked low vitamin D status with COVID-19 outcomes; however, these results are contradictory: 2 retrospective studies reported independent associations between low pre-pandemic vitamin D levels and the subsequent incidence and severity of COVID-19 [19,20], while an analogous study in the United Kingdom did not support the potential link between vitamin D concentration and the risk of severe COVID-19 infection and mortality [21]. A recent meta-analysis integrating data from 8 observational studies reported an increased risk of community-acquired pneumonia in patients with a serum vitamin D concentration <20 ng/mL [22]. Some recent reviews hypothesized that vitamin D insufficiency may compromise respiratory immune function, increasing the risk of COVID-19 [1,23]. Some retrospective studies have also observed possible correlations of vitamin D levels with COVID-19 outcomes [21,24–29]. Several studies have investigated the effect of vitamin D supplementation on COVID-19 outcomes [2] and evaluated the risk of developing COVID-19 infection in vitamin D-deficient patients and those with normal vitamin D levels [30,31], indicating the possible existence of a link between vitamin D levels and the risk of COVID-19 infection. However, the available data continue to be an area of uncertainty and an ongoing focus of attention [30].
- Low vitamin D levels are associated with comorbidities that are known to affect COVID-19 outcomes. Further investigations should focus on patients with low vitamin D levels with or without comorbidities and supplementation trials to investigate the effects of vitamin D on the immune response to COVID-19. Therefore, in the present systematic review, we aimed to determine the association of lower levels of vitamin D and comorbidities with the risk of COVID-19 infection. We hypothesized that lower vitamin D levels in the blood would be linked to a higher risk of COVID-19 infection.
INTRODUCTION
- The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines for systematic reviews [32] and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines [33] were followed for designing, conducting, and reporting this systematic literature review. The protocol of this systematic review was registered in PROSPERO (registration ID: CRD42020205150).
- Data Sources and Searches
- Three independent reviewers conducted the search for studies in the MEDLINE (via PubMed), Cochrane Central Register of Controlled Trials and ClinicalTrials.gov until August 20, 2021, using merged Medical Subject Headings (MeSH) and non-MeSH terms as follows: “vitamin D” OR “25-hydroxy vitamin D” AND “COVID-19” OR “2019 novel coronavirus” OR “SARS CoV-2”. We also searched the gray literature using Google Scholar and manually checked the reference lists of eligible articles.
- Inclusion and Exclusion Criteria
- Studies reporting the levels of vitamin D and comorbid conditions in COVID-19-positive and COVID-19-negative patients were included. We excluded reviews, editorials, opinions, case reports, case-series, perspectives, letters, protocols, and studies not reporting the required data. The first author (PM) searched data and screened articles for eligibility. The senior author (RP) double-checked all the included articles, and any dispute was resolved by the third author (RB).
- Quality Assessment
- Two reviewers (PM and RP) assessed the quality of data in the included studies using the National Institute of Health (NIH) quality assessment tools developed by the National Heart, Lung, and Blood Institute (NHLBI) [34]. The NIH tool was preferred because it is comprehensive and widely accepted for an exhaustive assessment of data quality. The tools were designed to assist reviewers in focusing on concepts that are key for critical appraisal of the internal validity of a study. The tools were not designed to provide a list of factors comprising a numeric score. The tools were specific to individual types of included study designs and are described in more detail below. The tools included items for evaluating potential flaws in study methods or implementation, including sources of bias (e.g., patient selection, performance, attrition, and detection), confounding, study power, the strength of causality in the association between interventions and outcomes, and other factors. The quality reviewers could select “yes,” “no,” or “cannot determine/not reported/not applicable” in response to each item on the tool. For each item where “no” was selected, reviewers were instructed to consider the potential risk of bias that could be introduced by that flaw in the study design or implementation. Responses of “cannot determine” and “not reported” were also noted as representing potential flaws. Each of the quality assessment tools had a detailed guidance document, which was also developed by the methodology team and NHLBI.
- Data Extraction
- Data were entered into a standardized data extraction table (Excel) and independently checked by a second reviewer (RP) for accuracy. The following raw data were extracted: name of the first author, year of publication, country of origin, study design, age, sex, identified comorbidities, reported levels of vitamin D (in the form of mean and standard deviation), patients’ socioeconomic status in terms of the Townsend deprivation quintile [21], and the number of patients in the COVID-19-positive and COVID-19 negative groups. The included studies designated reverse-transcription polymerase chain reaction (RT-PCR)–confirmed patients as COVID-19 positive and RT-PCR negative patients as COVID-19 negative groups. The patients were categorized as vitamin D-deficient or having low vitamin D levels if their vitamin D levels were <10 ng/mL (<25 nmol/L) [21,35], <20 ng/mL (<50 nmol/L) [5,26,36–43], <12 ng/mL (<30 nmol/L) [44,45] or <30 ng/mL (<75 nmol/L) [46,47]. Comorbidities described using words or phrases such as “hypertension” and “high blood pressure,” were classified as using the term “hypertension.” Comorbidities described as “chronic obstructive pulmonary disease,” “COPD,” or “chronic lung diseases” were referred to as “chronic lung disorder” in our study.
- Data Synthesis
- We performed an exploratory meta-analysis to understand the magnitude and direction of the effect estimate. Continuous outcomes are presented using weighted mean differences (MDs) and 95% confidence intervals (CIs). Odds ratios (ORs) were calculated and presented with respective 95% CIs for binary outcomes. The Mantel-Haenszel method for binary outcomes and the inverse-variance method for continuous outcomes were used to calculate 95% CIs. A random-effects model with the DerSimonian-Laird method was used to pool effect estimates, as substantial methodological heterogeneity was observed when pooling effect estimates [48]. Heterogeneity between studies was assessed using the chi-square-based Cochran Q statistic (with p<0.1 considered as indicating the presence of heterogeneity) and the I2 statistic (with >50% representing moderate heterogeneity) [48]. Publication bias was assessed only for the primary outcome by a visual inspection of a funnel plot, as the requirement for the minimum number of studies (≥10 studies) was satisfied. The Egger regression test was applied to assess small-study effect (with p<0.1 considered as indicating the presence of the small-study effect). We also used the Duval and Tweedie trim-and-fill method to estimate what the summary effect size would be if there was no publication bias [49]. A subgroup analysis was conducted if vitamin D deficiency was reported in proportions. We also analyzed the corresponding data if the mean vitamin D levels were reported by sex. We also calculated 95% prediction intervals where 3 or more studies were available; these intervals represent the direction and range of an effect estimate in a new study [50]. All statistical analyses were conducted using Stata version 17.0 (StataCorp., College Station, TX, USA), and a p-value less than 0.05 was considered a statistically significant result.
- Ethics Statement
- As the present study was a meta-analysis, the data was extracted from the published articles. Therefore, institutional review board approval was not required.
METHODS
- Study Characteristics
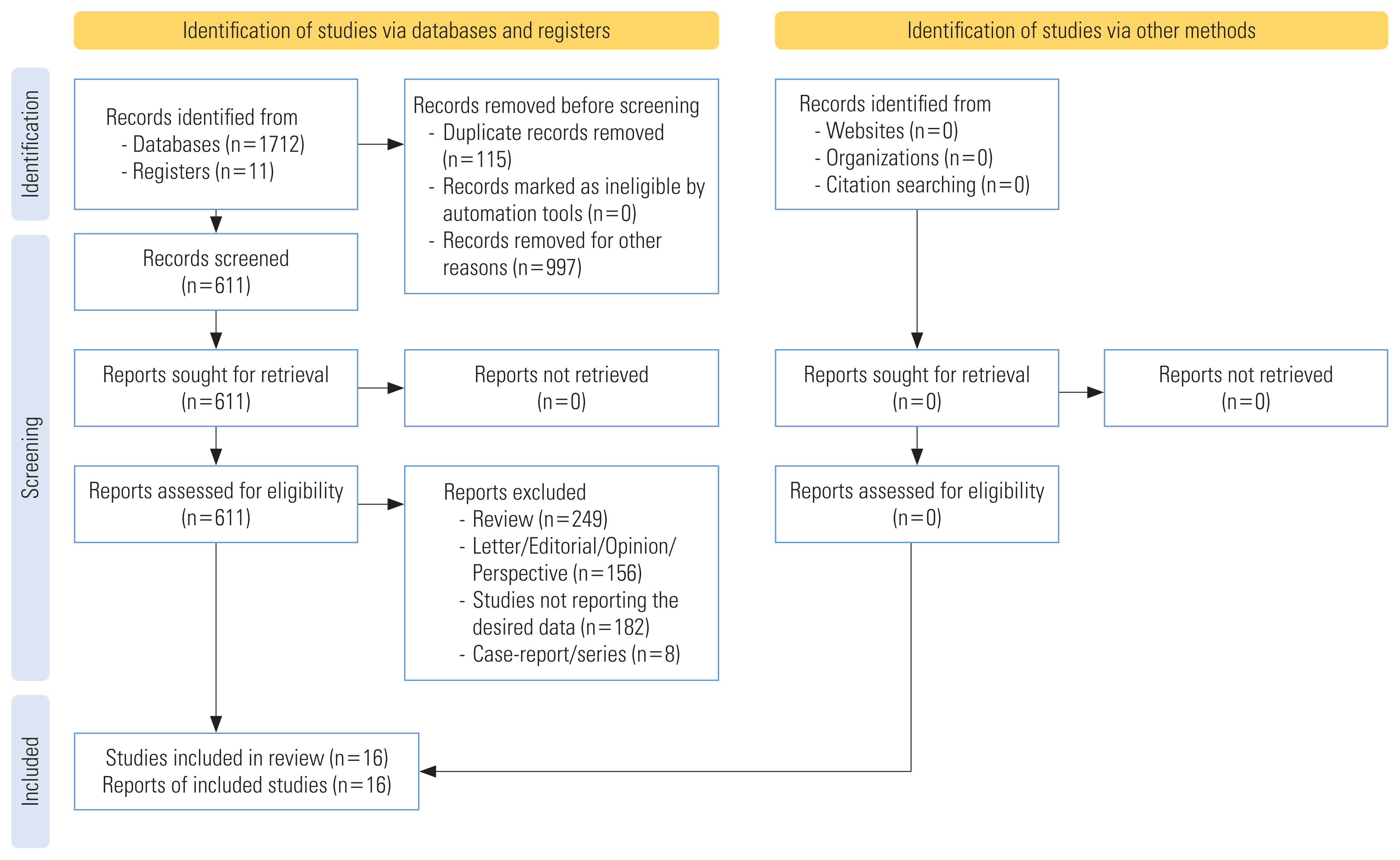
- The systematic search yielded a total of 1723 publications. After removing duplicates, 611 articles were found to be potential publications for screening. Studies were published between January 1, 2020 and August 20, 2021. After applying predefined inclusion and exclusion criteria, a total of 16 studies were included in the qualitative and quantitative analyses (Figure 1). The included studies were cohort studies [20,21, 25,26,39,40,44–46,51] and case-control studies [35–38,41,52]. The included 16 studies enrolled a total of 386 631 patients from 4 different countries, including 181 114 male patients and 205 517 female patients. The background characteristics of the included studies are presented in Table 1.
- Eight of the included articles [21,35–38,41,46,51] presented specific comorbidity data, in which the 4 most prevalent comorbidities were diabetes, cardiovascular disease, respiratory diseases and hypertension. The most common cardiovascular diseases were found to be coronary artery disease and hypercholesterolemia, while chronic lung disorder was the most prevalent respiratory disease. We also included the Townsend deprivation index in our analysis as a comorbidity.
- Quality Assessment
- We assessed the quality of data in the included studies using the NIH quality assessment tools, and presented the results in Table 1. The majority of the included studies (nearly 67%) were of acceptable quality. All the papers clearly stated the research question or objective, the study population was clearly specified and defined, and all the patients were selected from the same or similar populations. The detailed results of the quality assessment are provided in Supplemental Material 1.
- Association Between Vitamin D Levels and COVID-19
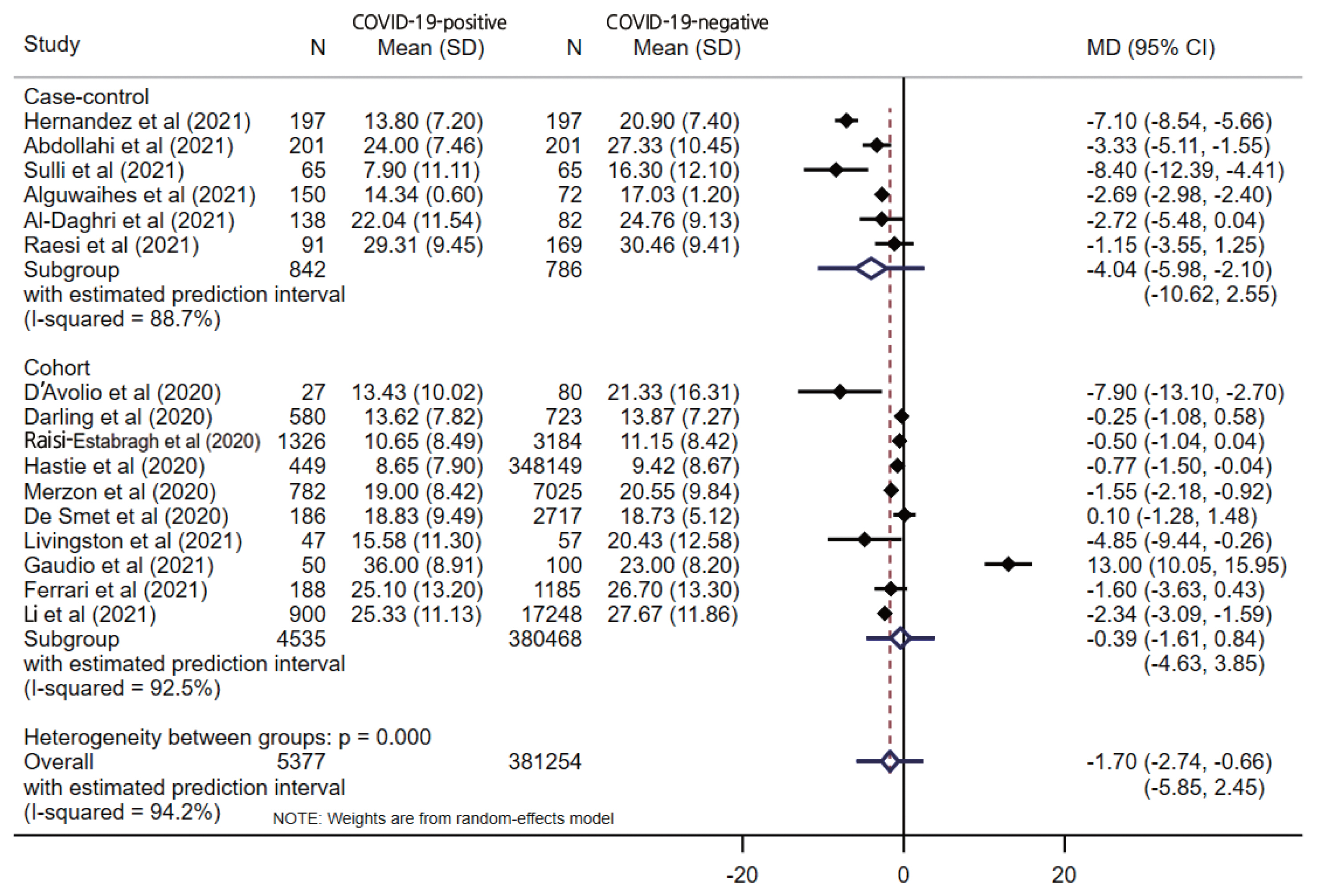
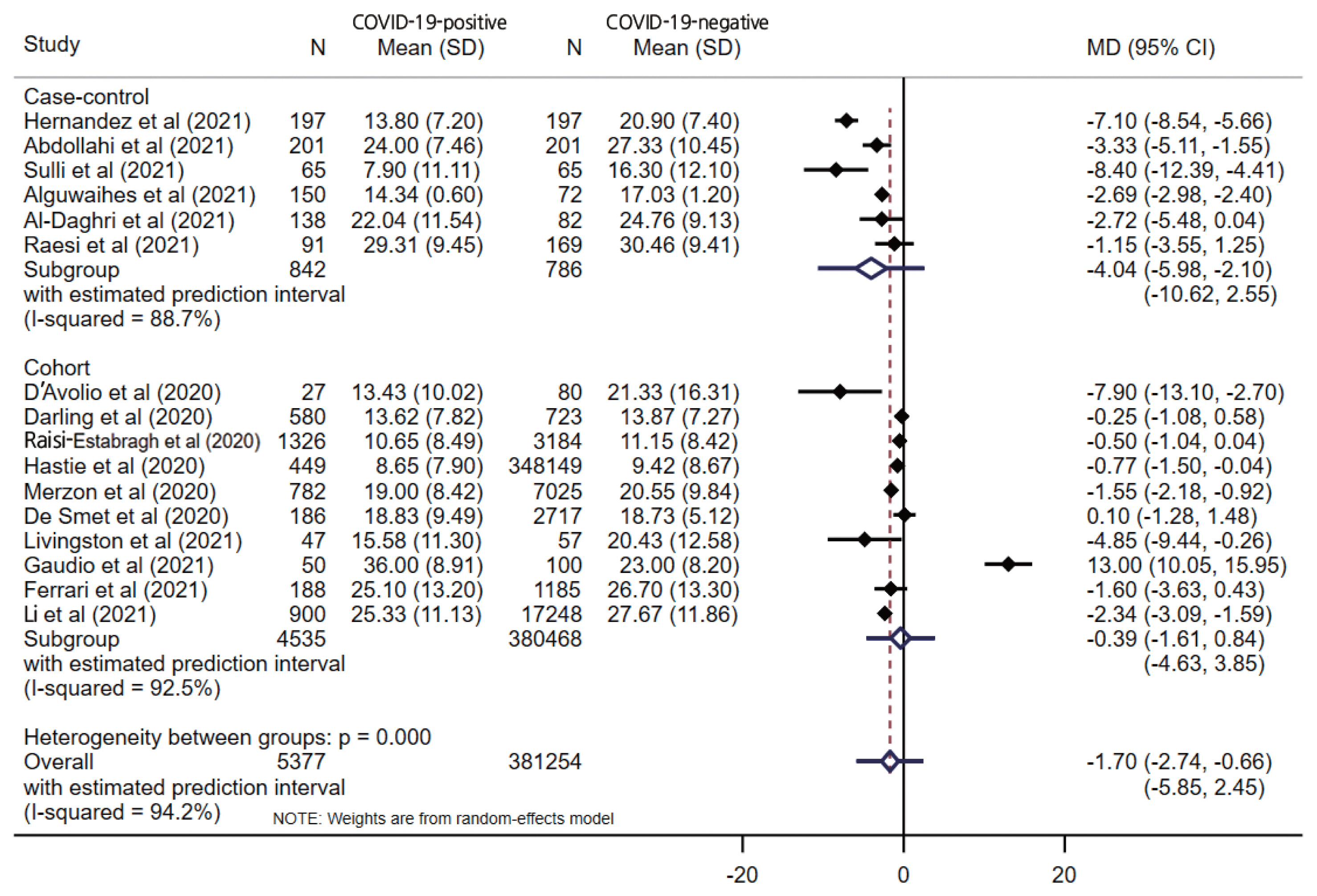
- The pooled estimate from 16 studies showed significantly lower serum levels of vitamin D in COVID-19-positive patients (MD, −1.70; 95% CI, −2.74 to −0.66; p=0.001) with a 95% prediction interval of −5.85 to 2.45 (Figure 2). When stratified by the study design, only the case-control design (MD, −4.04; 95% CI, −5.98 to −2.10; p<0.001) showed a significant difference between the COVID-19-positive and COVID-19-negative patients, unlike cohort studies (MD, −0.39; 95% CI −1.62 to 0.84; p=0.538). Substantial overall statistical heterogeneity (I2=94.2%; Cochran Q test p<0.001) was observed between the studies. Four studies reported vitamin D deficiency by categorizing vitamin D levels. The pooled OR for vitamin D deficiency in COVID-19-positive patients was 1.61 (95% CI, 1.20 to 2.17; I2=56.3%; p= 0.002) compared to COVID negative patients, with a 95% prediction interval of 0.53 to 4.94.
- Subgroup Analysis
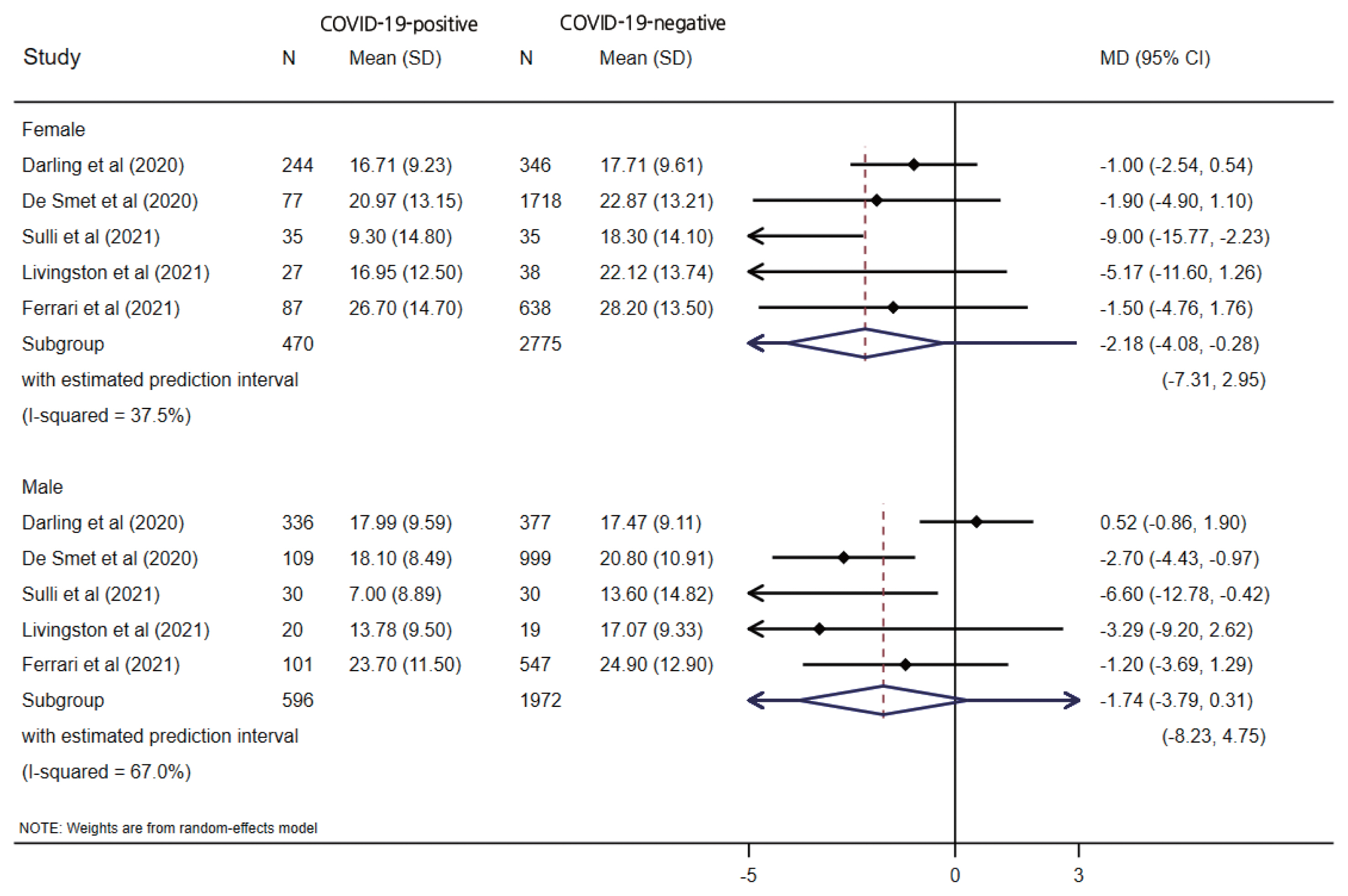
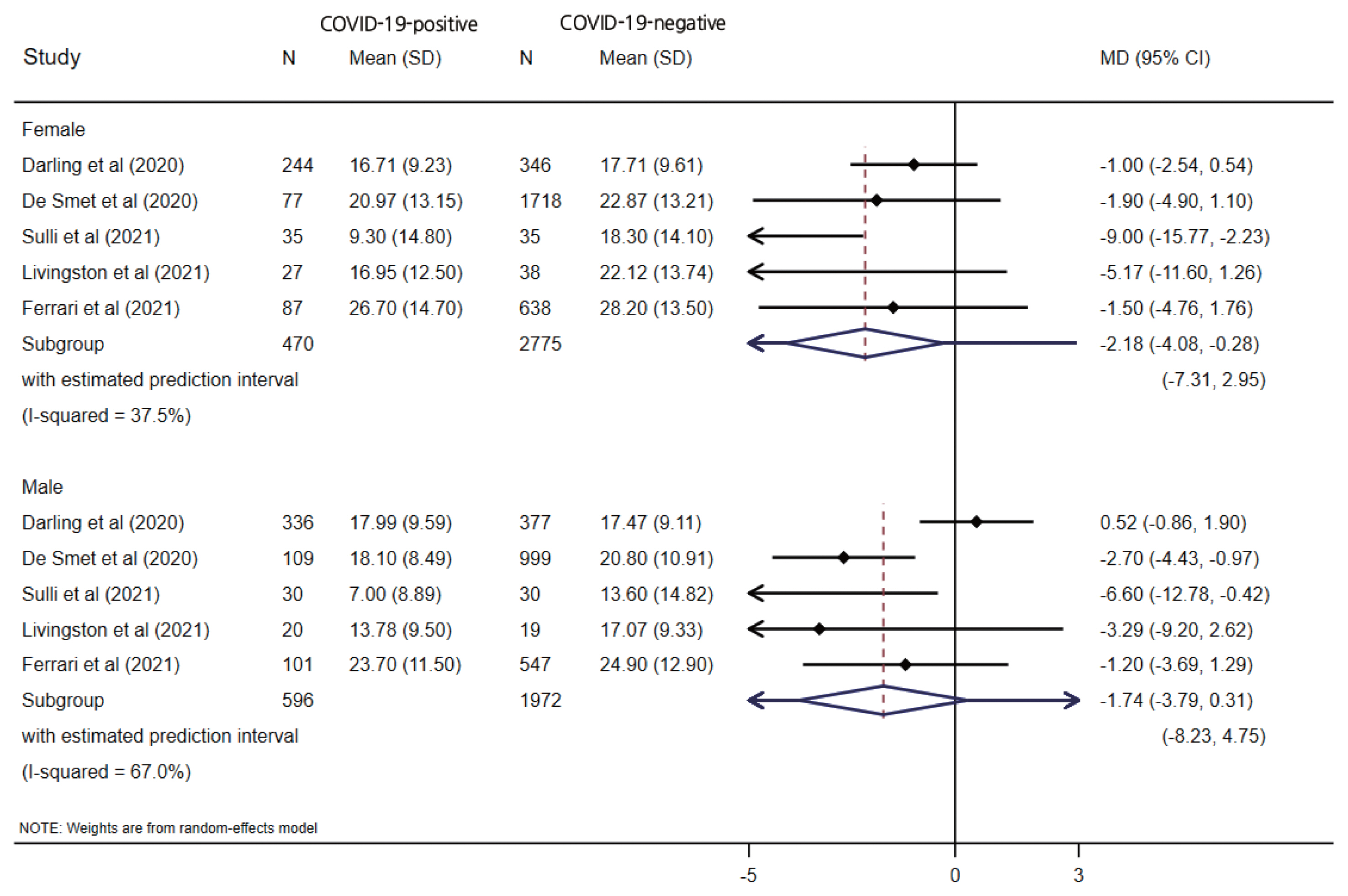
- Five studies reported vitamin D levels by sex (Figure 3). In a subgroup analysis based on sex, the difference in serum vitamin D levels was larger between female COVID-19 patients and female controls (MD, −2.18; 95% CI, −4.08 to −0.28; p=0.024; I2=37.5%) than the corresponding difference between male patients and controls (MD, −1.74; 95% CI, −3.79 to 0.31; p=0.096; I2=67.0%), with wide prediction intervals for both estimates. One study reported the proportion of vitamin D deficiency (vitamin D <20 ng/mL), and male patients showed higher odds of having low vitamin D levels (OR, 2.09; 95% CI, 1.38 to 3.17; p<0.001) than female patients (OR, 1.17; 95% CI 0.74 to 1.86; p=0.477). When stratified by the study design, only a case-control design (MD, −4.04; 95% CI, −5.98 to −2.10; p<0.001) showed a significant difference between the COVID-19-positive and COVID-19-negative patients, unlike the cohort studies (MD, −0.39; 95% CI, −1.62 to 0.84; p=0.538), which had wider prediction intervals, as shown in Figure 2.
- Comorbidities and Acquiring COVID-19 Infection
- A pooled analysis of the data from the aforementioned studies showed that patients exposed to comorbidities such as obesity (OR, 1.74; 95% CI, 1.43 to 2.11; p<0.001) and Townsend deprivation quintile 5 (OR, 2.00; 95% CI, 1.64 to 2.44; p<0.001) had higher odds of acquiring COVID-19 infection. However, patients with schizophrenia (OR, 0.56; 95% CI, 0.37 to 0.82; p= 0.003), coronary artery disease (OR, 0.64; 95% CI, 0.46 to 0.87; p=0.005), dementia (OR, 0.56; 95% CI, 0.43 to 0.71; p<0.001), Townsend deprivation quintile 1 (OR, 0.62; 95% CI, 0.47 to 0.81; p=0.001), Townsend deprivation quintile 3 (OR, 0.65; 95% CI, 0.50 to 0.85; p=0.002) had lower odds of acquiring COVID-19 infection. Furthermore, other comorbidities such as diabetes, hypertensions, chronic lung disease, and cardiovascular disease showed no statistically significant associations with COVID-19 infection (Table 2).
- Publication Bias
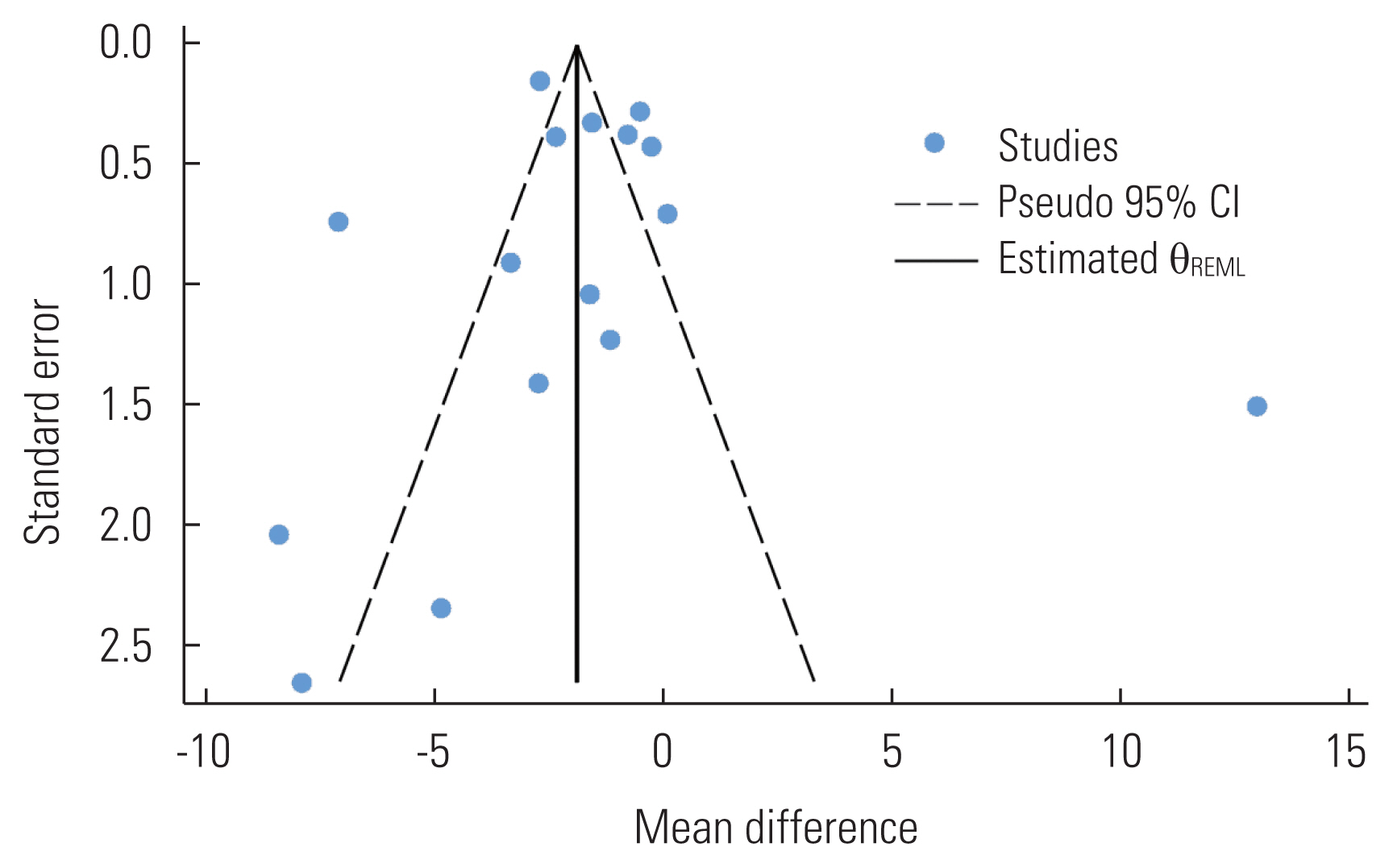
- Publication bias was assessed for the primary outcome (i.e., overall vitamin D levels) by visual inspection of a funnel plot and the Egger test. We clearly observed evidence of publication bias in the funnel plot, as studies were oddly distributed across the effect line (Figure 4). However, we did not observe statistical evidence for the small-study effect (p=0.651). The trim-and-fill analysis did not show any significant change in the effect estimate (p=0.358).
RESULTS
- The present systematic review and meta-analysis included 16 studies with a total of 5377 COVID-19-positive patients to study the association of lower levels of vitamin D and comorbidities with the risk of COVID-19 infection. Through an analysis of the available data, we found a significant association of vitamin D deficiency with the risk of COVID-19 infection [19, 26,53–57]. A possible cause for this observation may be related to vitamin D’s role in a variety of the body’s immune responses. Vitamin D, also known as calcitriol, is the active form of vitamin D. Upon interacting with the vitamin D receptor (VDR), which is found on immune cells (B, T, and antigen-presenting cells) and pulmonary epithelial cells, the calcitriol-VDR complex induces transcriptional expression of antimicrobial peptides, such as cathelicidins and defensins. Cathelicidins disrupt bacterial cell membranes, as well as enveloped viruses, such as SARS-CoV-2, while defensins promote chemotaxis of inflammatory cells via increased capillary permeability. Despite the fact that vitamin D promotes the expression of several inflammatory cytokines through T-cell inactivation and interferon activation, it also inhibits the pro-inflammatory markers interleukin-6 and tumor necrosis factor-α, which are 2 key cytokines involved in the development of the “cytokine storm” that precedes acute respiratory stress disorder. Given this molecular understanding, it is reasonable to believe that individuals with vitamin D deficiency are at a high risk of developing more severe COVID-19 symptoms and/or a worse prognosis [58].
- For infectious diseases caused by viruses, there are abundant and diverse ways in which sex can impact differential susceptibility between males and females. Although many studies have addressed the sex discrepancy in COVID-19, very few reports have analyzed the underlying cause of this disparity [59,60]. Several studies have analyzed levels of vitamin D in COVID-19 patients according to sex [61–63]; however, the reported findings are contradictory. We performed a subgroup analysis based on sex and observed a stronger tendency for female COVID-19 patients to have lower levels of vitamin D than COVID-19-negative patients (MD, −2.18; 95% CI, −4.08 to −0.28; p=0.024) than was the case for male patients (MD, −1.74; 95% CI, −3.79 to 0.31; p=0.096). Male patients showed higher odds of having low vitamin D levels (OR, 2.09; 95% CI, 1.38 to 3.17; p<0.001) than female patients (OR, 1.17; 95% CI, 0.74 to 1.86; p=0.477).
- Vitamin D deficiency has been shown to be a risk factor for COVID-19, especially for severe/critical cases [64]. Various retrospective observational studies have demonstrated an association of vitamin D deficiency with COVID-19 risk [19,26,65] and have suggested the usefulness of vitamin D supplementation to reduce the risk of infection [18,20,26,64]. Elderly individuals and people with comorbidities are more susceptible to severe COVID-19 infection and may demonstrate worse morbidity outcomes [66–68]. Since the risk of symptomatic upper respiratory tract infection has been suggested to be associated with low vitamin D levels, its concentrations are expected to be quite low in COVID-19-positive patients [20]. Guan et al. [69] reported comorbidities and their impacts on 1590 COVID-19 patients, and indicated that 399 (25.1%) patients reported at least 1 comorbidity, including hypertension, cardiovascular disease, cerebrovascular disease, diabetes, hepatitis B infection, chronic obstructive pulmonary disease, chronic kidney disease, malignancy, and immunodeficiency. Subsequently, Wang et al. [70] reported findings from 138 cases of COVID-19; the results suggested that comorbidities may be risk factors for adverse outcomes. In our study, we found that obesity and socioeconomic status were major risk factors for COVID-19 infection, which may be supported by the findings of meta-analyses performed to investigate the association of obesity and socioeconomic status in COVID-19 patients [71,72]. Assessing the prevalence of chronic diseases forms the basis for mitigating complications in patients with COVID-19. However, these efforts were hampered by the limited number of cases in the earliest stages of the pandemic [73].
- Compared with previous meta-analyses [53–57], our study had a larger sample size, making the results more credible. Furthermore, an analysis of vitamin D levels by sex was not performed in previous studies. The evidence presented in this review shows promise for the use of vitamin D supplementation to reduce the risk and severity of COVID-19 infection.
- To our knowledge, this meta-analysis included the highest number of COVID-19 patients not treated with any vitamin D supplementation. The present meta-analysis demonstrated a significant association between COVID-19 positivity and vitamin D levels in case-control studies, while no association was found in cohort studies. The reason for the difference in the results may be due to differences in the study design; although observational studies are more prone to bias, it was still seen that the case-control study design showed different results from those of cohort studies. This meta-analysis of observational studies provides a general idea of the association between vitamin D levels and COVID-19. Hence, further randomized studies are recommended to be conducted to assess the effect of vitamin D on COVID-19. The present study has some limitations: (1) there are discrepancies in the number and sample size of the included studies, leading to some instances of large variance in effect size estimates; and (2) significant heterogeneity was found, and we only used random-effects models to address heterogeneity, which may have affected the strength and extrapolation of conclusions; (3) publication bias may have affected our results because negative studies were less likely to be published; and (4) although we conducted an extensive search, we may have inadvertently missed some relevant studies.
DISCUSSION
- Low serum vitamin D levels were significantly associated with a high risk of acquiring COVID-19 infection; however, the results varied by study design. This relationship was more prominent in female patients than in male patients. Limited evidence was found regarding relationships between reported comorbidities and COVID-19 infection; therefore, large population-based studies are recommended to establish any association. Vitamin D-deficient individuals should be provided special attention. Vitamin D levels can be monitored and supplementation of vitamin D could be considered in patients to improve their recovery if they contract COVID-19.
CONCLUSION
SUPPLEMENTAL MATERIALS
-
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
-
FUNDING
None.
Notes
ACKNOWLEDGEMENTS
-
AUTHOR CONTRIBUTIONS
Conceptualization: Mishra P, Parveen R. Data curation: Mishra P, Parveen R. Formal analysis: Mishra P, Bajpai R. Funding acquisition: None. Methodology: Mishra P, Bajpai R. Project administration: Agarwal N, Mishra P. Visualization: Mishra P, Parveen R. Writing – original draft: Mishra P, Parveen R, Agarwal N, Bajpai R. Writing – review & editing: Bajpai R, Agarwal N.
Notes

| Study | Study design | Country | Groups | Sample size | Age, mean±SD or median (IQR)/range y | Sex, male (%) | Definition of vitamin D status | Levels of vitamin D, mean±SD or median (IQR/range) | Comorbidities | Quality Index |
|---|---|---|---|---|---|---|---|---|---|---|
| D’Avolio et al., 2020 [20] | Retrospective cohort | Switzerland | COVID-19 positive | 27 | 74 (65–81) | 19 (70.4) | NR | 11.1 (8.2–21.0) | NA | Fair |
| COVID-19 negative | 80 | 73 (61–82) | 39 (48.8) | 24.6 (8.9–30.5) | ||||||
|
|
||||||||||
| Darling et al., 2020 [25] | Prospective cohort | UK | COVID-19 positive | 580 | 57.5 (8.7) | 336 (57.5) | NR | 43.3 (32.1) | NA | Fair |
| COVID-19 negative | 723 | 57.9 (8.7) | 377 (52.1) | 44.1 (31.2) | ||||||
|
|
||||||||||
| Raisi-Estabragh et al., 2020 [51] | Prospective cohort | UK | COVID-19 positive | 1326 | 68.11±9.23 | 696 (52.5) | NR | 33.88±27.01 | Diabetes, hypertension, high cholesterol | Good |
| COVID-19 negative | 3184 | 68.91±8.72 | 1,505 (47.3) | 35.45±26.78 | ||||||
|
|
||||||||||
| Hastie et al., 2020 [21] | Retrospective cohort | UK | COVID-19 positive | 449 | 49 (40–58) | 265 (59.0) | Deficiency (<25 nmol/L), Insufficiency (<50 nmol/L) | 28.7 (10.0–43.8) | Diabetes | Good |
| COVID-19 negative | 348 149 | 49 (38–57) | 168 391 (48.4) | 32.7 (10.0–47.2) | ||||||
|
|
||||||||||
| Merzon et al., 2020 [46] | Retrospective cohort | Israel | COVID-19 positive | 782 | 68.11±9.23 | 385 (49.2) | Low (<30 ng/mL) | 19.00 (18.41–19.59) | Diabetes, hypertension, high cholesterol, depression, schizophrenia, dementia, cardiovascular disease, coronary artery disease, chronic lung disorder | Good |
| COVID-19 negative | 7025 | 68.91±8.72 | 397 (50.8) | 20.55 (20.32–20.78) | ||||||
|
|
||||||||||
| De Smet et al., 2020 [26] | Retrospective cohort | Belgium | COVID-19 positive | 186 | 69 (52–80) | 109 (58.6) | Deficiency (<20 ng/mL) | 18.6 (12.6–25.3) | Cardiovascular disease, coronary artery disease, | Good |
| COVID-19 negative | 2717 | 68 (49–82) | 999 (36.8) | 21.5 (13.9–20.8) | ||||||
|
|
||||||||||
| Hernández et al., 2021 [38] | Retrospective case-control | Spain | COVID-19 positive | 197 | 61 (47.5–70) | 123 (62.4) | Deficiency (<20 ng/mL) | 13.8±7.2 | Hypertension, diabetes, cardiovascular disease, chronic lung disease | Fair |
| COVID-19 negative | 197 | 61 (56–66) | 123 (62.4) | 20.9±7.4 | ||||||
|
|
||||||||||
| Abdollahi et al., 2021 [35] | Case-control | Iran | COVID-19 positive | 201 | 24 (19–29) | 66 | Deficient (<10 ng/mL), insufficient (10–30 ng/mL), sufficient (>30–100 ng/mL) | 24 (19–29) | Hypertension, diabetes, chronic lung disease | Fair |
| COVID-19 negative | 201 | 26 (21–35) | 66 | Sufficiency (>30 ng/mL) | 26 (21–35) | |||||
|
|
||||||||||
| Sulli et al., 2021 [41] | Case-control | Italy | COVID-19 positive | 65 | 7.9 (15) | 30 (46.2) | Insufficiency (between 20 and 30 ng/mL), Deficiency (between 10 and 20 ng/mL), and severe deficiency (<10 ng/mL) | 7.9 (15) | Hypertension, diabetes, cardiovascular disease, coronary artery disease, chronic lung disease | Fair |
| COVID-19 negative | 65 | 16.3 (19) | 30 (46.2) | 16.3 (19) | ||||||
|
|
||||||||||
| Alguwaihes et al., 2021 [36] | Retrospective case-control | Saudi Arabia | COVID-19 positive | 150 | 35.8±1.5 | 97 (64.7) | Deficiency (<50 nmol/L) and severe deficiency (<12.5 nmol/L) | 35.8±1.5 | Hypertension, diabetes, cardiovascular disease | Fair |
| COVID-19 negative | 72 | 42.5±3.0 | 38 (52.8) | 42.5±3.0 | ||||||
|
|
||||||||||
| Livingston et al., 2021 [45] | Retrospective cohort | UK | COVID-19 positive | 47 | 38.9±28.2 | 20 (42.6) | Deficiency (<30 nmol/L) | 38.9±28.2 | NA | Fair |
| COVID-19 negative | 57 | 51.0±31.4 | 19 (33.3) | 51.0±31.4 | ||||||
|
|
||||||||||
| Gaudio et al., 2021 [44] | Retrospective cohort | Italy | COVID-19 positive | 50 | 12.5 (2–42) | 26 (52.0) | Deficiency <12 ng/mL (30 nmol/L) and insufficiency 12–20 ng/mL (50 nmol/L) | 12.5 (2–42) | NA | Good |
| COVID-19 negative | 100 | 20.5 (5–46) | 44 (44.0) | 20.5 (5–46) | ||||||
|
|
||||||||||
| Ferrari et al., 2021 [39] | Retrospective cohort | Italy | COVID-19 positive | 188 | 25.1±13.2 | 101 (53.7) | Deficiency (<20 ng/mL) | 25.1±13.2 | NA | Fair |
| COVID-19 negative | 1185 | 26.7±13.3 | 547 (46.1) | 26.7±13.3 | ||||||
|
|
||||||||||
| Al-Daghri et al., 2021 [37] | Case-control | Saudi Arabia | COVID-19 positive | 138 | 55.0±28.8 | 79 (57.2) | Deficiency (<50 nmol/L) | 55.0±28.8 | Hypertension, diabetes, high cholesterol, cardiovascular disease | Fair |
| COVID-19 negative | 82 | 61.8±22.8 | 41 (50.0) | 61.8±22.8 | ||||||
|
|
||||||||||
| Li et al., 2021 [40] | Cohort | USA | COVID-19 positive | 900 | 25 (18–33) | 252 (28.0) | Levels below 20 or 30 ng/mL as low | 25 (18–33) | NA | Good |
| COVID-19 negative | 17 248 | 27 (20–36) | 5726 (33.2) | 27 (20–36) | ||||||
|
|
||||||||||
| Raesi et al., 2021 [52] | Case-control | Iran | COVID-19 positive | 91 | 73.16±23.59 | 55 (60.4) | NR | 73.16±23.59 | Hypertension, diabetes, high cholesterol, cardiovascular disease, chronic lung disorder | Fair |
| COVID-19 negative | 169 | 76.02±23.48 | 113 (66.9) | 76.02±23.48 | ||||||
- 1. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020;12(4):988ArticlePubMedPMC
- 2. Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM 2021;114(3):175-181ArticlePubMedPMCPDF
- 3. Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr 2020;74(6):856-859ArticlePubMedPMCPDF
- 4. Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015;7(10):8251-8560ArticlePubMedPMC
- 5. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 2017;18(2):153-165ArticlePubMedPDF
- 6. Dankers W, Colin EM, van Hamburg JP, Lubberts E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol 2017;7: 697ArticlePubMedPMC
- 7. Infante M, Ricordi C, Sanchez J, Clare-Salzler MJ, Padilla N, Fuenmayor V, et al. Influence of vitamin D on islet autoimmunity and beta-cell function in type 1 diabetes. Nutrients 2019;11(9):2185ArticlePubMedPMC
- 8. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40(4):1109-1151ArticlePubMedPMC
- 9. Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect 2006;134(6):1129-1140ArticlePubMedPMC
- 10. Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E. On the epidemiology of influenza. Virol J 2008;5: 29ArticlePubMedPMC
- 11. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 2009;169(6):626-632ArticlePubMedPMC
- 12. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020;12(1):236ArticlePubMedPMC
- 13. Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem 2010;285(4):2227-2231PubMed
- 14. Panagiotou G, Tee SA, Ihsan Y, Athar W, Marchitelli G, Kelly D, et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf) 2020;93(4):508-511ArticlePubMedPMCPDF
- 15. Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr 2011;106(9):1433-1440ArticlePubMed
- 16. Kralj M, Jakovac H. Vitamin D and COVID-19 in an immunocompromised patient with multiple comorbidities-a case report. Clin Case Rep 2021;9(4):2269-2275ArticlePubMedPMCPDF
- 17. Parveen R, Sehar N, Bajpai R, Agarwal NB. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract 2020;166: 108295ArticlePubMedPMC
- 18. Mishra P, Parveen R, Agarwal NB. Role of vitamin D in risk reduction of COVID-19: a narrative review. Ann Natl Acad Med Sci (India) 2021;57(1):36-40Article
- 19. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D deficiency and treatment with COVID-19 incidence. MedRxiv [Preprint] 2020. [cited 2021 Sep 10]. Available from: https://doi.org/10.1101/2020.05.08.20095893 Article
- 20. D’Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020;12(5):1359ArticlePubMedPMC
- 21. Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr 2020;14(4):561-565ArticlePubMedPMC
- 22. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore) 2019;98(38):e17252PubMedPMC
- 23. Cutolo M, Paolino S, Smith V. Evidences for a protective role of vitamin D in COVID-19. RMD Open 2020;6(3):e001454ArticlePubMedPMC
- 24. Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. MedRxiv [Preprint]; 2020 [cited 2021 Oct 2]. Available from: https://doi.org/10.1101/2020.04.08.20058578 Article
- 25. Darling AL, Ahmadi KR, Ward KA, Harvey NC, Alves AC, Dunn-Walters DK, et al. Vitamin D status, body mass index, ethnicity and COVID-19: initial analysis of the first-reported UK Biobank COVID-19 positive cases (n 580) compared with negative controls (n 723). MedRxiv [Preprint] 2020. [cited 2021 Oct 2]. Available from: https://doi.org/10.1101/2020.04.29.20084277 Article
- 26. De Smet DD, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Vitamin D deficiency as risk factor for severe COVID-19: a convergence of two pandemics. MedRxiv [Preprint] 2020. [cited 2021 Oct 2]. Available from: https://doi.org/10.1101/2020.05.01.20079376 Article
- 27. Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020;32(7):1195-1198ArticlePubMedPMCPDF
- 28. Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, et al. Vitamin D insufficiency is prevalent in severe COVID-19. MedRxiv [Preprint] 2020. [cited 2021 Oct 2]. Available from: https://doi.org/10.1101/2020.04.24.20075838 Article
- 29. Li Y, Li Q, Zhang N, Liu Z. Sunlight and vitamin D in the prevention of coronavirus disease (COVID-19) infection and mortality in the United States. Res Sq [Preprint] 2020. [cited 2021 Oct 2]. Available from: https://doi.org/10.21203/rs.3.rs-32499/v1 Article
- 30. Teshome A, Adane A, Girma B, Mekonnen ZA. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health 2021;9: 624559ArticlePubMedPMC
- 31. Crafa A, Cannarella R, Condorelli RA, Mongioì LM, Barbagallo F, Aversa A, et al. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: a systematic review and meta-analysis. EClinicalMedicine 2021;37: 100967ArticlePubMedPMC
- 32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71ArticlePubMedPMC
- 33. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283(15):2008-2012ArticlePubMed
- 34. National Heart, Lung, and Blood Institute. Study quality assessment tools; [cited 2020 Jun 9]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 35. Abdollahi A, Kamali Sarvestani H, Rafat Z, Ghaderkhani S, Mahmoudi-Aliabadi M, Jafarzadeh B, et al. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: a case-control study of hospitalized patients in Tehran, Iran. J Med Virol 2021;93(4):2359-2364ArticlePubMedPDF
- 36. Alguwaihes AM, Sabico S, Hasanato R, Al-Sofiani ME, Megdad M, Albader SS, et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case-control study in an Arab Gulf country. Aging Clin Exp Res 2021;33(5):1415-1422ArticlePubMedPMCPDF
- 37. Al-Daghri NM, Amer OE, Alotaibi NH, Aldisi DA, Enani MA, Sheshah E, et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: a multi-centre case-control study. J Transl Med; 2021 19(1):166ArticlePubMedPMCPDF
- 38. Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab 2021;106(3):e1343-e1353ArticlePubMedPMCPDF
- 39. Ferrari D, Locatelli M, Faraldi M, Lombardi G. Changes in 25-(OH) vitamin D levels during the SARS-CoV-2 outbreak: lockdown-related effects and first-to-second wave difference-an observational study from northern Italy. Biology (Basel) 2021;10(3):237ArticlePubMedPMC
- 40. Li Y, Tong CH, Bare LA, Devlin JJ. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw Open 2021;4(5):e2111634ArticlePubMedPMC
- 41. Sulli A, Gotelli E, Casabella A, Paolino S, Pizzorni C, Alessandri E, et al. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 2021;13(3):717ArticlePubMedPMC
- 42. Al-Daghri NM, Al-Saleh Y, Aljohani N, Sulimani R, Al-Othman AM, Alfawaz H, et al. Vitamin D status correction in Saudi Arabia: an experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch Osteoporos 2017;12(1):1ArticlePubMedPMCPDF
- 43. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 2019;180(4):P23-P54ArticlePubMed
- 44. Gaudio A, Murabito AR, Agodi A, Montineri A, Castellino P. D O CoV Research, Vitamin D levels are reduced at the time of hospital admission in Sicilian SARS-CoV-2-positive patients. Int J Environ Res Public Health 2021;18(7):3491PubMedPMC
- 45. Livingston M, Plant A, Dunmore S, Hartland A, Jones S, Laing I, et al. Detectable respiratory SARS-CoV-2 RNA is associated with low vitamin D levels and high social deprivation. Int J Clin Pract 2021;75(7):e14166ArticlePubMedPMCPDF
- 46. Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J 2020;287(17):3693-3702ArticlePubMedPMCPDF
- 47. Wirral Clinical Commissioning Group. Treatment of vitamin D deficiency in adults; 2020 [cited 2022 Jul 19]. Available from: https://mm.wirral.nhs.uk/document_uploads/guidelines/Vitamin%20D%20Guidelines%20for%20Adults%20v2.pdf
- 48. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions; 2011 [cited 2022 Jul 19]. Available from: https://www.radioterapiaitalia.it/wp-content/uploads/2017/01/cochrane-handbook-for-systematic-reviews-of-interventions.pdf
- 49. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56(2):455-463ArticlePubMed
- 50. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6(7):e010247ArticlePubMedPMC
- 51. Raisi-Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;42(3):451-460ArticlePubMedPMCPDF
- 52. Raesi A, Saedi Dezaki E, Moosapour H, Saeidifard F, Habibi Z, Rahmani F, et al. Hypocalcemia in Covid-19: a prognostic marker for severe disease. Iran J Pathol 2021;16(2):144-153ArticlePubMedPMC
- 53. Szarpak L, Rafique Z, Gasecka A, Chirico F, Gawel W, Hernik J, et al. A systematic review and meta-analysis of effect of vitamin D levels on the incidence of COVID-19. Cardiol J 2021;28(5):647-654ArticlePubMedPMC
- 54. Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and vItamin D (VIVID): a systematic review and meta-analysis. Metabolism 2021;119: 154753ArticlePubMedPMC
- 55. Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis 2021;104: 58-64ArticlePubMedPMC
- 56. Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr 2022;62(5):1308-1316ArticlePubMed
- 57. Ghasemian R, Shamshirian A, Heydari K, Malekan M, Alizadeh-Navaei R, Ebrahimzadeh MA, et al. The role of vitamin D in the age of COVID-19: a systematic review and meta-analysis. Int J Clin Pract 2021;75(11):e14675ArticlePubMedPMCPDF
- 58. Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol 2021;93(2):733-740ArticlePubMedPDF
- 59. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8(4):e20ArticlePubMedPMC
- 60. Wenham C, Smith J, Morgan R; Gender COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet 2020;395(10227):846-848ArticlePubMedPMC
- 61. Muscogiuri G, Barrea L, Somma CD, Laudisio D, Salzano C, Pugliese G, et al. Sex differences of vitamin D status across BMI classes: an observational prospective cohort study. Nutrients 2019;11(12):3034ArticlePubMedPMC
- 62. Sanghera DK, Sapkota BR, Aston CE, Blackett PR. Vitamin D status, gender differences, and cardiometabolic health disparities. Ann Nutr Metab 2017;70(2):79-87ArticlePubMedPMCPDF
- 63. Pagano MT, Peruzzu D, Ruggieri A, Ortona E, Gagliardi MC. Vitamin D and sex differences in COVID-19. Front Endocrinol (Lausanne) 2020;11: 567824ArticlePubMedPMC
- 64. Ye K, Tang F, Liao X, Shaw BA, Deng M, Huang G, et al. Does serum vitamin D level affect COVID-19 infection and its severity?-A case-control study. J Am Coll Nutr 2021;40(8):724-731ArticlePubMed
- 65. Murdaca G, Pioggia G, Negrini S. Vitamin D and Covid-19: an update on evidence and potential therapeutic implications. Clin Mol Allergy 2020;18(1):23ArticlePubMedPMCPDF
- 66. Baktash V, Hosack T, Patel N, Shah S, Kandiah P, Van den Abbeele K, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J 2021;97(1149):442-447ArticlePubMedPMC
- 67. Campi I, Gennari L, Merlotti D, Mingiano C, Frosali A, Giovanelli L, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis 2021;21(1):566ArticlePubMedPMCPDF
- 68. Kweder H, Eidi H. Vitamin D deficiency in elderly: risk factors and drugs impact on vitamin D status. Avicenna J Med 2018;8(4):139-146ArticlePubMedPMC
- 69. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55(5):2000547ArticlePubMedPMC
- 70. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061-1069ArticlePubMedPMC
- 71. Ho JS, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: a systematic review and meta-analysis. Ann Acad Med Singap 2020;49(12):996-1008ArticlePubMed
- 72. Mamelund SE, Shelley-Egan C, Rogeberg O. The association between socioeconomic status and pandemic influenza: systematic review and meta-analysis. PLoS One 2021;16(9):e0244346ArticlePubMedPMC
- 73. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94: 91-95ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Effects of vitamin D on the incidence and severity of COVID-19
V. V. Krivosheev, L. Yu. Nikitina, I. V. Kozlovskiy, A. V. Fedorov
Sanitarnyj vrač (Sanitary Doctor).2024; (1): 27. CrossRef - Ukrainian Consensus on Diagnosis and Management of Vitamin D Deficiency in Adults
Nataliia Grygorieva, Mykola Tronko, Volodymir Kovalenko, Serhiy Komisarenko, Tetiana Tatarchuk, Ninel Dedukh, Mykola Veliky, Serhiy Strafun, Yulia Komisarenko, Andrii Kalashnikov, Valeria Orlenko, Volodymyr Pankiv, Oleg Shvets, Inna Gogunska, Svitlana Reg
Nutrients.2024; 16(2): 270. CrossRef - Effect of Vitamin D3 Supplementation on Severe COVID-19: A Meta-Analysis of Randomized Clinical Trials
Marharyta Sobczak, Rafał Pawliczak
Nutrients.2024; 16(10): 1402. CrossRef - Methodological issues in designing and reporting of systematic reviews in assessing association between vitamin D supplementation and COVID-19 severity
R Bajpai
QJM: An International Journal of Medicine.2023; 116(5): 406. CrossRef - Mechanistic Insight into the role of Vitamin D and Zinc in Modulating Immunity Against COVID-19: A View from an Immunological Standpoint
Nuzhat Ahsan, Mohammad Imran, Yousuf Mohammed, Fatme Al Anouti, Mohammad Idreesh Khan, Tanushree Banerjee, Mohd Adnan, Fauzia Ashfaq, Marek Kieliszek, Syed Amir Ashraf, Afrozul Haq
Biological Trace Element Research.2023; 201(12): 5546. CrossRef - The Role of Diet and Specific Nutrients during the COVID-19 Pandemic: What Have We Learned over the Last Three Years?
Petra Rust, Cem Ekmekcioglu
International Journal of Environmental Research and Public Health.2023; 20(7): 5400. CrossRef - Self-Reported Pre-Pandemic Physical Activity and Likelihood of COVID-19 Infection: Data from the First Wave of the CoCo-Fakt Survey
Nikola Schmidt, Andreas Gehlhar, Barbara Grüne, Annelene Kossow, Thomas Kraus, Johannes Nießen, Stefanie Wessely, Christine Joisten
Sports Medicine - Open.2023;[Epub] CrossRef - Diagnosis, prevention and treatment of vitamin D deficiency in adults: Ukrainian experts consensus statement
N.V. Grygorieva, M.D. Tronko, V.M. Kovalenko, S.V. Komisarenko, T.F. Tatarchuk, N.V. Dedukh, M.M. Veliky, S.S. Strafun, Y.I. Komisarenko, A.V. Kalashnikov, V.L. Orlenko, V.I. Pankiv, O.V. Shvets, I.V. Gogunska, S.I. Regeda
PAIN, JOINTS, SPINE.2023; 13(2): 60. CrossRef - Vitamin D Deficiency and COVID-19: A Biological Database Study on Pathways and Gene-Disease Associations
Ángela Alcalá-Santiago, Miguel Rodríguez-Barranco, Marta Rava, María Ángeles Jiménez-Sousa, Ángel Gil, María José Sánchez, Esther Molina-Montes
International Journal of Molecular Sciences.2022; 23(22): 14256. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite