Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 45(6); 2012 > Article
-
Original Article
Association of Homocysteine Levels With Blood Lead Levels and Micronutrients in the US General Population - Yu-Mi Lee1, Mi-Kyung Lee2, Sang-Geun Bae1, Seon-Hwa Lee1, Sun-Young Kim3, Duk-Hee Lee1
-
Journal of Preventive Medicine and Public Health 2012;45(6):387-393.
DOI: https://doi.org/10.3961/jpmph.2012.45.6.387
Published online: November 29, 2012
1Department of Preventive Medicine, Kyungpook National University School of Medicine, Daegu, Korea.
2Department of Family Medicine, Kyungpook National University Hospital, Daegu, Korea.
3Department of Public Health, Kyungpook National University Graduate School, Daegu, Korea.
- Corresponding author: Duk-Hee Lee, MD, PhD. Gukchaebosang-ro, Jung-gu, Daegu 700-842, Korea. Tel: +82-53-420-4866, Fax: +82-53-425-2447, lee_dh@knu.ac.kr
Copyright © 2012 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- Even though several epidemiological studies have observed positive associations between blood lead levels and homocysteine, no study has examined whether this association differs by the levels of micronutrients, such as folate, vitamin B6, and vitamin B12, which are involved in the metabolism of homocysteine. In this study, we examined the interactions between micronutrients and blood lead on homocysteine levels.
-
Methods
- This study was performed with 4089 adults aged ≥20 years old in the US general population using the National Health and Nutrition Examination Survey 2003-2004.
-
Results
- There were significant or marginally significant interactions between micronutrients and blood lead levels on mean homocysteine levels. Positive associations between blood lead and homocysteine were clearly observed among subjects with low levels of folate or low vitamin B6 (p-trend <0.01, respectively). However, in the case of vitamin B12, there was a stronger positive association between blood lead and homocysteine among subjects with high levels of vitamin B12, compared to those with low levels of vitamin B12. In fact, the levels of homocysteine were already high among subjects low in vitamin B12, irrespective of blood lead levels. When we used hyperhomocysteinemia (homocysteine>15 µmol/L) as the outcome, there were similar patterns of interaction, though p-values for each interaction failed to reach statistical significance.
-
Conclusions
- In the current study, the association between blood lead and homocysteine differed based on the levels of folate, vitamin B6, or vitamin B12 present in the blood. It may be important to keep sufficient levels of these micronutrients to prevent the possible harmful effects of lead exposure on homocysteine levels.
- Elevated homocysteine is known to be an independent risk factor for cardiovascular disease [1,2]. Since widespread arteriosclerosis in patients with homocystinuria, which is caused by deficiencies of enzymes involved in homocysteine metabolism, was first reported in 1969 [3], many clinical and epidemiological researches have shown that an elevated homocysteine level is a potent independent risk factor for arteriosclerosis in the general population [1]. For example, a meta-analysis of observational studies indicated that increased homocysteine levels were a modest independent predictor of coronary heart disease and cerebrovascular disease risk in healthy populations [2]. Although controversy exists regarding a causal relationship, increased homocysteine levels have also been linked with impaired cognitive function [4,5].
- High levels of homocysteine are linked to genetic mutations (e.g., 677C→T mutation in 5,10-methylenetetrahydrofolate reductase [MTHFR]), physiological factors (e.g., increasing age, male gender, and postmenopausal state), lifestyle factors (e.g., smoking), deficiency of micronutrients (e.g., folate, vitamin B6, and vitamin B12), and some clinical conditions (e.g., renal failure, hypothyroidism, and late stage of diabetes) [6,7].
- On the other hand, several recent studies have reported a positive relationship between blood lead and homocysteine levels in the general population or lead-exposed workers, suggesting that heavy metals like lead can disturb the metabolism of homocysteine [8-11]. Lead, in particular, can disturb essential enzymes in the metabolism of homocysteine like cystathionine β-synthase or MTHFR [12-14]. Notably, micronutrients such as folate, vitamin B6, and vitamin B12 are also involved in the pathways of homocysteine metabolism. Therefore, there is a possibility of interaction between blood lead levels and these micronutrients on the levels of homocysteine.
- However, to the best of our knowledge, there has been no study exploring this hypothesis. Thus, we examined whether the association between blood lead and homocysteine differs depending on the levels of micronutrients like folate, vitamin B6, and vitamin B12 in a general population.
INTRODUCTION
- Study Population
- The National Health and Nutrition Examination Survey (NHANES), carried out annually by the Centers for Disease Control and Prevention, is a complex, multi-stage probability research designed to measure the health and nutritional status of the non-institutionalized US general population [15]. In this study, data from NHANES 2003-2004 were used. Among the 5041 participants aged 20 years or older in NHANES 2003-2004, 4089 subjects with valid measured values of plasma homocysteine, whole blood lead, vitamin B6, serum folate, and vitamin B12 levels were included in this study.
- Measures
- The details of the NHANES protocol and all testing procedures are available elsewhere [15]. Demographic, socioeconomic, and health related characteristics were collected from detailed interview using the standardized questionnaire in participant's homes. Physiological measurements and laboratory tests were conducted by highly trained medical personnel in specially designed and equipped mobile centers.
- Venous blood samples were collected and shipped weekly at -20℃. Total homocysteine levels in plasma was determined by the Abbott Homocysteine assay on the Abbott AxSym analyzer, a fully automated fluorescence polarization immunoassay obtained from Abbott Diagnostics (Abbott Laboratories, Abbott Park, IL, USA). Whole blood lead concentration was measured using inductively coupled plasma mass spectrometry, a multi-element analytical technique [16]. Both serum folate and vitamin B12 levels were determined by using the Bio-Rad Laboratories "Quantaphase II Folate/Vitamin B12" radioassay kit (Bio-Rad Laboratories, Hercules, CA, USA). Plasma pyridoxal 5'-phosphate (PLP), the biologically active form of vitamin B6, was measured using the Enzymatic B6 Assay (A/C Diagnostics, San Diego, CA, USA).
- Statistical Analysis
- Blood lead levels were categorized into quartiles (Q1 to Q4) by cutoff points at the 25th, 50th, and 75th percentile values. Because the range of the highest quartile level (Q4) was too wide, it was sub-divided into four additional categories (Q4-1 to Q4-4), making seven categories of blood lead levels, in total.
- First, we evaluated the associations between blood lead levels and homocysteine using a generalized linear model. Also, using logistic regression analysis, we calculated adjusted odds ratios (ORs) for hyperhomocysteinemia (level of plasma homocysteine>15 µmol/L) according to the categories of blood lead levels. Possible confounders were age (years, continuous), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), and poverty income ratio (PIR; an index calculated by family income divided by the poverty threshold, specific to family size, continuous). Briefly, a PIR of less than 1 indicates a family income below the poverty threshold; a PIR of more than 1 indicates a family income one or more times the poverty threshold, body mass index (BMI; kg/m2, continuous), smoking status (never, former, or current), alcohol consumption (none or current), and physical activity (inactive, moderate, or vigorous). Just as previous studies have done [8,11], we further considered as confounders serum folate (ng/mL, continuous), vitamin B12 (pg/mL, continuous), and plasma vitamin B6 (nmol/L, continuous).
- Second, we calculated the associations of blood lead levels with continuous homocysteine or dichotomous hyperhomocysteinemia stratified by median levels of serum folate, vitamin B6, and vitamin B12 to determine whether the associations differed depending on micronutrient levels. All statistical analyses were conducted with SAS version 9.2 (SAS Inc., Cary, NC, USA) and SUDAAN version 9.0 (RTI International, Research Triangle Park, NC, USA). Instead of using sample weights, estimates of main results were calculated accounting for stratification and clustering [17], adjusting for age, sex, race/ethnicity, and PIR; this adjustment is considered a good compromise between efficiency and bias [17,18]. The results from SAS and from SUDAAN were very similar, so we present the results based on SAS. A p-value of less than 0.05 was considered statistically significant.
METHODS
- General Characteristics of the Study Subjects According to the Categories of Blood Lead Levels
- The sample of 4089 subjects had a mean age of 50.4±19.3 years old. The mean BMI was 28.4±6.2 kg/m2 and mean PIR was 2.6±1.6. Mean values of serum folate, vitamin B6, and vitamin B12 were 14.2±15.1 ng/mL, 67.4±82.4 nmol/L, and 555.2±846.6 pg/mL, respectively. The study subjects were 48.7% male, 53.8% non-Hispanic white, 18.5% current smokers, 71.4% current drinkers, and 41.4% physically inactive people. Subjects with high blood lead levels tended to be older, male, less obese, non-Hispanic white, current smokers, current drinkers, and have a lower PIR and be more physically inactive (p-trend <0.001 for all these variables) compared with those with low blood lead levels (Table 1).
- Associations of Blood Lead Levels With Homocysteine Levels or Hyperhomocysteinemia
- Homocysteine levels showed a positive trend with blood lead levels. In the unadjusted analysis, mean homocysteine levels according to the categories of blood lead levels were 7.5, 9.1, 10.0, 10.1, 11.0, 11.2, and 12.2 µmol/L, respectively (p-trend <0.001) (Table 2). This linear relationship between homocysteine and blood lead remained statistically significant after adjusting for covariates including age, sex, race/ethnicity, PIR, BMI, smoking, alcohol consumption, and physical activity. However, the magnitude of the increase was smaller than the unadjusted analysis: 9.2, 9.7, 9.8, 9.6, 10.1, 10.3, and 11.2 µmol/L, respectively (p-trend <0.001). Further adjustment for serum folate, vitamin B6, and vitamin B12 did not change the results.
- When we used the dichotomous outcome of hyperhomocysteinemia, the unadjusted ORs were 1.0, 2.1, 3.3, 4.3, 4.1, 8.3, and 11.1 across the categories of blood lead levels (p-trend <0.001) (Table 2). After all covariates were adjusted, the ORs for hyperhomocysteinemia were 1.0, 1.2, 1.3, 1.6, 1.2, 2.6, and 3.2 (p-trend <0.001).
- These patterns were similarly observed among subjects without chronic diseases, specifically coronary artery disease, cerebral vascular disease, hypertension, diabetes, cancers, thyroid disease, and kidney disease, which could influence homocysteine levels (data not shown).
- Stratified Analyses by the Level of Serum Folate, Vitamin B6, or Vitamin B12
- When study subjects were stratified according to the median value of serum folate, vitamin B6, or vitamin B12, the positive association between blood lead levels and homocysteine levels were more clearly observed among subjects with lower folate (p-trend <0.001) or lower vitamin B6 (p-trend <0.001) (Table 3). There was a stronger positive association between blood lead and homocysteine among subjects with high vitamin B12 than those with low vitamin B12 (p-trend <0.001); and subjects with low vitamin B12 already had high levels of homocysteine, irrespective of blood lead levels. A p-value for interaction was statistically significant or marginally significant for all three micronutrients.
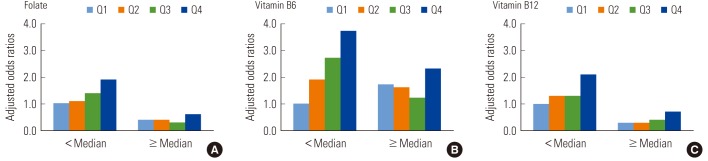
- There were similar patterns in general when we used hyperhomocysteinemia as the outcome, though p-values for interactions failed to reach statistical significance (Table 3). Namely, clear and statistically significant increasing trends were observed only among subjects with lower folate (p-trend 0.003) or lower vitamin B6 (p-trend <0.001). In the case of vitamin B12, the positive association tended to be observed among subjects with both lower and higher vitamin B12 (p-trend 0.012 and 0.005, respectively). We also presented the adjusted ORs, using subjects with blood lead levels in the 1st quartile and micronutrients less than the median value as the reference group, to give a comprehensive view on the combination effect of lead and each micronutrient (Figure 1).
RESULTS
- Our current study showed a linear association between blood lead and homocysteine among the US general population even though 95.2% of study subjects had blood lead levels less than 5 µg/dL. In addition, as we hypothesized, the association between lead and homocysteine differed depending on serum folate, vitamin B6, and vitamin B12 levels.
- The association between blood lead and homocysteine is consistent with the findings of several previous studies [8-11]. For example, in the Baltimore Memory Study, a population-based study of adults aged 50 to 70 years old, homocysteine levels increased 0.35 µmol/L per every 1.0 µg/dL increase in blood lead [9]. In a study of Vietnamese factory workers occupationally exposed to lead, an elevation of 1.0 µg/dL in blood lead was associated with an elevation of 0.05 µmol/L in homocysteine on the logarithmic scale [10]. Two other general population studies, one of US adults aged 20 to 59 years old who participated in the NHANES and the other of Pakistani adults aged 18 to 60 years old, also showed an increase of homocysteine as blood lead levels increased [8,11]. Based on our findings and those of other studies, it could be suggested that blood lead levels could be another predictor of hyperhomocysteinemia.
- Importantly, we observed statistically significant interactions between blood lead and the micronutrients folate, vitamin B6, and vitamin B12, which are involved in transmethylation or transsulfuration metabolism on homocysteine levels. The positive association between blood lead and homocysteine was mainly observed when the levels of folate or vitamin B6 were low while there was no clear association among subjects with high levels of folate or vitamin B6. On the other hand, unlike folate or vitamin B6, in the case of vitamin B12, the positive association was observed only among subjects with high vitamin B12 levels. In fact, homocysteine levels were already substantially high among subjects with low vitamin B12 levels irrespective of blood lead levels. If homocysteine levels increase above certain levels, additional synergic effects of blood lead levels may be negligible.
- Meanwhile, although the p-values of interactions between blood lead levels and micronutrients on the blood concentrations of homocystein were marginally significant or significant, those in a logistic regression using a dichotomous variable for hyperhomocysteinemia as an outcome, failed to reach statistical significance. As the continuous homocysteine levels tested additive interactions while the dichotomous hyperhomocysteinemia tested multiplicative interactions, more significant results in the additive interactions can be expected. Even though the multiplicative interactions failed to reach statistical significances, it is notable that the patterns of interactions were similarly observed in both models.
- Homocysteine is a sulfur-containing amino acid derived from the metabolism of methionine, and can be metabolized by one of two pathways: 1) transsulfuration to cysteine, and 2) remethylation to methionine [19]. In the transsulfuration pathway, homocysteine is transformed to cystathionine by the enzyme cystathionine β-synthase with vitamin B6, which is a PLP-dependent heme-containing enzyme [19,20]. Because lead toxicity inhibits heme and hemoglobin synthesis [21], this could be one mechanism that explains the observed association between blood lead and homocysteine.
- In addition, in the remethylation pathway, homocysteine can be remethylated to methionine through the enzyme methionine synthase with vitamin B12, folate, and MTHFR [19]. At the same time, it has been suggested that the variant enzyme MTHFR might cause lead to bind more to active sites, which may result in the impairment of enzyme function [14]. On the other hand, the heavy metals, including lead, have high electron-sharing affinities for the free sulfhydryl group of proteins [21,22]. As homocysteine itself contains a sulfhydryl group, blood lead may inhibit the metabolism of homocysteine by this pathway. Taken together, there is a possibility that lead might directly inactivate the enzymes involved in methionine metabolism, or might adhere competitively to the active site for homocysteine, or homocysteine itself. Lead can also biologically interact with folate, vitamin B6, and vitamin B12.
- Current study has some limitations. First, in this cross-sectional study we cannot determine the causality between blood lead and homocysteine, despite the biological plausibility discussed above. Second, we could not consider gene mutation, which is already known to be associated with the level of homocysteine (e.g., point mutation of enzyme MTHFR 677C→T). It is difficult to imagine that blood lead levels would be different depending on the mutation of enzyme MTHFR. Therefore, gene mutation may not act as a strong confounder in this study. However, further studies on the effect of genetic polymorphisms of enzymes involved in homocysteine metabolism would be helpful to understand the current findings.
- Regardless of these limitations, the current study has several strengths. First, this study employed the strict sampling design, large sample size, and controlled quality of study measurements used in NHANES. Second, to best of our knowledge, this is the first study to investigate the interaction between the level of blood lead and the micronutrients involved in the metabolism of homocysteine in estimating the level of serum homocysteine.
- In conclusion, we found that mean homocysteine levels and the risk of hyperhomocysteinemia increased with blood lead levels. Importantly, these relationships differed by the level of the micronutrients involved in the metabolism of homocysteine. It may be important to maintain sufficient levels of these micronutrients to prevent possible lead exposure from harming the metabolism of homocysteine.
DISCUSSION
ACKNOWLEDGEMENTS
- 1. McCully KS. Hyperhomocysteinemia and arteriosclerosis: historical perspectives. Clin Chem Lab Med 2005;43(10):980-986. 16197285ArticlePubMed
- 2. Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002;288(16):2015-2022. 12387654ArticlePubMed
- 3. McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969;56(1):111-128. 5792556PubMedPMC
- 4. Vogel T, Dali-Youcef N, Kaltenbach G, Andrès E. Homocysteine, vitamin B12, folate and cognitive functions: a systematic and critical review of the literature. Int J Clin Pract 2009;63(7):1061-1067. 19570123ArticlePubMed
- 5. Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, et al. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry 2011;19(7):607-617. 21705865ArticlePubMed
- 6. Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50(1):3-32. 14709635ArticlePubMed
- 7. Ganji V, Kafai MR. Third National Health and Nutrition Examination Survey. Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr 2003;77(4):826-833. 12663279ArticlePubMed
- 8. Yakub M, Iqbal MP. Association of blood lead (Pb) and plasma homocysteine: a cross sectional survey in Karachi, Pakistan. PLoS One 2010;5(7):e11706. 20657730ArticlePubMedPMC
- 9. Schafer JH, Glass TA, Bressler J, Todd AC, Schwartz BS. Blood lead is a predictor of homocysteine levels in a population-based study of older adults. Environ Health Perspect 2005;113(1):31-35. 15626644ArticlePubMedPMC
- 10. Chia SE, Ali SM, Lee BL, Lim GH, Jin S, Dong NV, et al. Association of blood lead and homocysteine levels among lead exposed subjects in Vietnam and Singapore. Occup Environ Med 2007;64(10):688-693. 17449564ArticlePubMedPMC
- 11. Krieg EF Jr, Butler MA. Blood lead, serum homocysteine, and neurobehavioral test performance in the third National Health and Nutrition Examination Survey. Neurotoxicology 2009;30(2):281-289. 19459225ArticlePubMed
- 12. Bar-Or D, Rael LT, Thomas GW, Kraus JP. Inhibitory effect of copper on cystathionine beta-synthase activity: protective effect of an analog of the human albumin N-terminus. Protein Pept Lett 2005;12(3):271-273. 15777277ArticlePubMed
- 13. Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS, et al. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry 2004;9(4):358-370. 14745455ArticlePubMed
- 14. Yakub M, Moti N, Parveen S, Chaudhry B, Azam I, Iqbal MP. Polymorphisms in MTHFR, MS and CBS genes and homocysteine levels in a Pakistani population. PLoS One 2012;7(3):e33222. 22470444ArticlePubMedPMC
- 15. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: NHANES 2003-2004. cited 2012 May 7. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/nhanes03_04.htm
- 16. Date AR, Gray AL. Applications of Inductively Coupled Plasma Mass Spectrometry. 1989. New York: Chapman and Hall; p. 1-38
- 17. Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health 1991;81(9):1166-1173. 1951829ArticlePubMedPMC
- 18. Graubard BI, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst 1999;91(12):1005-1016. 10379963ArticlePubMed
- 19. Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: causal relationship and potential mechanisms. Biofactors 2009;35(2):120-129. 19449439ArticlePubMed
- 20. Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J 2001;20(15):3910-3916. 11483494ArticlePubMedPMC
- 21. Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 2008;128(4):501-523. 19106443PubMed
- 22. Quig D. Cysteine metabolism and metal toxicity. Altern Med Rev 1998;3(4):262-270. 9727078PubMed
REFERENCES
| Characteristics |
Quartiles of blood lead levels |
p-trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 4089) | Q1 (n = 971) | Q2 (n = 1100) | Q3 (n = 1001) |
Q4 |
|||||
| Q4-1 (n = 252) | Q4-2 (n = 242) | Q4-3 (n = 270) | Q4-4 (n = 253) | ||||||
| Mean±standard deviation | |||||||||
| Blood lead (μg/dL) | 2.1±1.8 (0.2-33.0) | 0.8±0.2 (0.2-1.0) | 1.3±0.2 (1.1-1.6) | 2.1±0.3 (1.7-2.5) | 2.7±0.1 (2.6-2.9) | 3.2±0.1 (3.0-3.4) | 3.9±0.3 (3.5-4.5) | 7.2±3.7 (4.6-33.0) | |
| Age (y) | 50.4±19.3 | 38.7±16.9 | 48.1±18.4 | 55.3±17.7 | 56.4±17.3 | 61.3±17.0 | 59.5±18.0 | 59.7±18.5 | <0.001 |
| Body mass index (kg/m2) | 28.4±6.2 | 29.1±7.6 | 28.5±5.9 | 28.7±5.8 | 27.8±5.6 | 27.5±4.6 | 27.2±5.2 | 27.4±5.3 | <0.001 |
| Poverty income ratio | 2.6±1.6 | 2.7±1.6 | 2.7±1.6 | 2.6±1.6 | 2.5±1.6 | 2.4±1.5 | 2.1±1.5 | 2.0±1.4 | <0.001 |
| Serum folate (ng/mL) | 14.2±15.1 | 14.5±10.5 | 14.5±23.2 | 14.2±12.4 | 14.2±8.9 | 14.1±8.0 | 13.4±8.6 | 12.2±9.3 | 0.03 |
| Vitamin B6 (nmol/L) | 67.4±82.4 | 60.7±80.5 | 69.3±78.2 | 75.3±95.2 | 61.1±53.3 | 73.0±91.8 | 62.8±68.7 | 59.5±78.5 | 0.98 |
| Vitamin B12 (pg/mL) | 555.2±846.6 | 511.9±429.8 | 522.4±281.7 | 569.4±688.3 | 721.4±2244.2 | 517.9±293.1 | 636.8±1605.9 | 591.7±887.5 | 0.009 |
| Proportion (%) | |||||||||
| Male | 48.7 | 24.8 | 46.6 | 55.0 | 62.7 | 66.5 | 65.6 | 75.5 | <0.001 |
| Non-Hispanic white | 53.8 | 60.8 | 54.5 | 51.5 | 52.8 | 53.3 | 49.6 | 39.5 | <0.001 |
| Current smokers | 18.5 | 10.9 | 16.9 | 19.0 | 25.4 | 23.1 | 26.7 | 33.2 | <0.001 |
| Current drinkers | 71.4 | 67.0 | 67.3 | 73.8 | 79.4 | 74.8 | 73.7 | 82.2 | <0.001 |
| Physically inactive | 41.4 | 31.9 | 38.0 | 43.1 | 48.4 | 47.5 | 52.2 | 60.9 | <0.001 |
| Folate deficiency1 | 0.4 | 0.1 | 0.5 | 0.3 | 1.6 | 0.4 | 0.4 | 1.2 | 0.03 |
| Vitamin B6 deficiency2 | 22.0 | 30.5 | 20.0 | 17.6 | 17.9 | 19.4 | 20.4 | 23.7 | <0.001 |
| Vitamin B12 deficiency3 | 2.2 | 2.1 | 2.2 | 2.2 | 0.8 | 1.7 | 3.7 | 3.6 | 0.17 |
Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile.
1 Model 1: unadjusted; Model 2: adjusted for age, sex, race/ethnicity, and poverty income ratio; Model 3: further adjusted for body mass index, smoking, alcohol, and physical activity; Model 4: further adjusted for serum folate, vitamin B6, and vitamin B12.
Q1, first quartile; Q2, second quartile; Q3, third quartile; Q4, fourth quartile; OR, odds ratio; CI, confidence interval.
1 Adjusted for age, sex, race/ethnicity, poverty income ratio, body mass index, smoking, alcohol, and physical activity.
2 Median values of serum folate, vitamin B6, and vitamin B12 are 11.6 ng/mL, 45.6 nmol/L, and 465.0 pg/mL, respectively.
Figure & Data
References
Citations

- Blood Homocysteine Levels Mediate the Association Between Blood Lead Levels and Cardiovascular Mortality
Sapha Shibeeb, Atiyeh Abdallah, Zumin Shi
Cardiovascular Toxicology.2024; 24(1): 62. CrossRef - Association of low-level blood lead with plasma homocysteine in US children and adolescents
Lingfei Shi, Jia Zhou, Jinjiang Dong, Faliang Gao, Wenyan Zhao
Environmental Geochemistry and Health.2023; 45(7): 5013. CrossRef - Prevalence of hyperhomocysteinemia (HHcy) and its major determinants among hypertensive patients over 35 years of age
Minna Cheng, Hong Xue, Xinjian Li, Qinghua Yan, Dingliang Zhu, Yan Wang, Yan Shi, Chen Fu
European Journal of Clinical Nutrition.2022; 76(4): 616. CrossRef - Maternal exposure to heavy metals and risk for severe congenital heart defects in offspring
Chengrong Wang, Xin Pi, Shengju Yin, Mengyuan Liu, Tian Tian, Lei Jin, Jufen Liu, Zhiwen Li, Linlin Wang, Zhengwei Yuan, Yu Wang, Aiguo Ren
Environmental Research.2022; 212: 113432. CrossRef - Lead (Pb) exposure and heart failure risk
Zihan Chen, Xia Huo, Guangcan Chen, Xiuli Luo, Xijin Xu
Environmental Science and Pollution Research.2021; 28(23): 28833. CrossRef - Plasma Vitamin B12 and Folate Alter the Association of Blood Lead and Cadmium and Total Urinary Arsenic Levels with Chronic Kidney Disease in a Taiwanese Population
Yu-Mei Hsueh, Ya-Li Huang, Yuh-Feng Lin, Horng-Sheng Shiue, Ying-Chin Lin, Hsi-Hsien Chen
Nutrients.2021; 13(11): 3841. CrossRef - Modification of vitamin B6 on the associations of blood lead levels and cardiovascular diseases in the US adults
Jia Wei, John S Ji
BMJ Nutrition, Prevention & Health.2020; 3(2): 180. CrossRef - Nonlinear association between blood lead and hyperhomocysteinemia among adults in the United States
Minghui Li, Lihua Hu, Wei Zhou, Tao Wang, Lingjuan Zhu, Zhenyu Zhai, Huihui Bao, Xiaoshu Cheng
Scientific Reports.2020;[Epub] CrossRef - Biomonitoring findings for occupational lead exposure in battery and ceramic tile workers using biochemical markers, alkaline comet assay, and micronucleus test coupled with fluorescence in situ hybridisation
Vilena Kašuba, Mirta Milić, Davor Želježić, Marin Mladinić, Alica Pizent, Zorana Kljaković-Gašpić, Melita Balija, Irena Jukić
Archives of Industrial Hygiene and Toxicology.2020; 71(4): 339. CrossRef - Exposure to Toxic Heavy Metals Can Influence Homocysteine Metabolism?
Caterina Ledda, Emanuele Cannizzaro, Piero Lovreglio, Ermanno Vitale, Angela Stufano, Angelo Montana, Giovanni Li Volti, Venerando Rapisarda
Antioxidants.2019; 9(1): 30. CrossRef - Single Nucleotide Polymorphisms in Key One-Carbon Metabolism Genes and Their Association with Blood Folate and Homocysteine Levels in a Chinese Population in Yunnan
Juan Ni, Yaoxian Liu, Tao Zhou, Xiayu Wu, Xu Wang
Genetic Testing and Molecular Biomarkers.2018; 22(3): 193. CrossRef - α-Tocopherol supplementation and the oxidative stress, homocysteine, and antioxidants in lead exposure
Sławomir Kasperczyk, Michał Dobrakowski, Aleksandra Kasperczyk, Ewa Nogaj, Marta Boroń, Zbigniew Szlacheta, Ewa Birkner
Archives of Environmental & Occupational Health.2017; 72(3): 153. CrossRef - Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: A population-based study and systematic review
Liyuan Han, Yanfen Liu, Changyi Wang, Linlin Tang, Xiaoqi Feng, Thomas Astell-Burt, Qi wen, Donghui Duan, Nanjia Lu, Guodong Xu, Kaiyue Wang, Lu Zhang, Kaibo Gu, Sihan Chen, Jianping Ma, Tao Zhang, Dingyun You, Shiwei Duan
Clinical Nutrition.2017; 36(5): 1215. CrossRef - Effect of occupational exposure to lead on new risk factors for cardiovascular diseases
Adam Prokopowicz, Andrzej Sobczak, Magdalena Szuła-Chraplewska, Marzena Zaciera, Jolanta Kurek, Izabela Szołtysek-Bołdys
Occupational and Environmental Medicine.2017; 74(5): 366. CrossRef - Blood lead, cadmium and mercury in relation to homocysteine and C-reactive protein in women of reproductive age: a panel study
Anna Z. Pollack, Sunni L. Mumford, Lindsey Sjaarda, Neil J. Perkins, Farah Malik, Jean Wactawski-Wende, Enrique F. Schisterman
Environmental Health.2017;[Epub] CrossRef - Blood lead levels, iron metabolism gene polymorphisms and homocysteine: a gene-environment interaction study
Kyoung-Nam Kim, Mee-Ri Lee, Youn-Hee Lim, Yun-Chul Hong
Occupational and Environmental Medicine.2017; 74(12): 899. CrossRef - Evaluation and management of lead exposure
Hwan-Cheol Kim, Tae-Won Jang, Hong-Jae Chae, Won-Jun Choi, Mi-Na Ha, Byeong-Jin Ye, Byoung-Gwon Kim, Man-Joong Jeon, Se-Yeong Kim, Young-Seoub Hong
Annals of Occupational and Environmental Medicine.2015;[Epub] CrossRef - Lead Exposure, B Vitamins, and Plasma Homocysteine in Men 55 Years of Age and Older: The VA Normative Aging Study
Kelly M. Bakulski, Sung Kyun Park, Marc G. Weisskopf, Katherine L. Tucker, David Sparrow, Avron Spiro, Pantel S. Vokonas, Linda Huiling Nie, Howard Hu, Jennifer Weuve
Environmental Health Perspectives.2014; 122(10): 1066. CrossRef
 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


