Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 47(2); 2014 > Article
-
Review
Environmental Mercury and Its Toxic Effects - Kevin M. Rice1, Ernest M. Walker2, Miaozong Wu1, Chris Gillette3, Eric R. Blough1,2,4
-
Journal of Preventive Medicine and Public Health 2014;47(2):74-83.
DOI: https://doi.org/10.3961/jpmph.2014.47.2.74
Published online: March 31, 2014
1Center for Diagnostic Nanosystems, Marshall University, Huntington, WV, USA.
2Department of Pharmacology, Physiology, and Toxicology, Joan C. Edwards School of Medicine, Marshall University, Huntington, WV, USA.
3Department of Pharmacy Practice, Administration, and Research, School of Pharmacy, Marshall University, Huntington, WV, USA.
4Department of Pharmaceutical Science and Research, School of Pharmacy, Marshall University, Huntington, WV, USA.
- Corresponding author: Eric R. Blough, PhD. Room #241 R, 1700, 3rd avenue, Huntington, WV 25755, USA. Tel: +1-304-696-2708, Fax: +1-304-696-5288, blough@marshall.edu
Copyright © 2014 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- INTRODUCTION
- STATES OF MERCURY
- SYSTEMIC TOXICOLOGICAL EFFECTS OF MERCURY
- CELLULAR EFFECTS OF MERCURY
- CARDIOVASCULAR, HEMATOLOGICAL, AND PULMONARY EFFECTS
- EFFECTS ON THE DIGESTIVE AND RENAL SYSTEMS
- EFFECTS ON THE IMMUNE SYSTEM
- EFFECTS ON THE NERVOUS SYSTEM
- EFFECTS ON THE ENDOCRINE SYSTEM
- EFFECTS ON THE REPRODUCTIVE SYSTEM
- FETOTOXICITY
- CONCLUSION
- Notes
- REFERENCES
ABSTRACT
- Mercury exists naturally and as a man-made contaminant. The release of processed mercury can lead to a progressive increase in the amount of atmospheric mercury, which enters the atmospheric-soil-water distribution cycles where it can remain in circulation for years. Mercury poisoning is the result of exposure to mercury or mercury compounds resulting in various toxic effects depend on its chemical form and route of exposure. The major route of human exposure to methylmercury (MeHg) is largely through eating contaminated fish, seafood, and wildlife which have been exposed to mercury through ingestion of contaminated lower organisms. MeHg toxicity is associated with nervous system damage in adults and impaired neurological development in infants and children. Ingested mercury may undergo bioaccumulation leading to progressive increases in body burdens. This review addresses the systemic pathophysiology of individual organ systems associated with mercury poisoning. Mercury has profound cellular, cardiovascular, hematological, pulmonary, renal, immunological, neurological, endocrine, reproductive, and embryonic toxicological effects.
- Mercury is ranked third by the US Government Agency for Toxic Substances and Disease Registry of the most toxic elements or substances on the planet to arsenic and lead that continues to be dumped into our waterways and soil, spilled into our atmosphere, and consumed in our food and water [1,2]. Human activities have nearly tripled the amount of mercury in the atmosphere and the atmospheric burden is increasing 1.5 percent per year [1]. Soil contaminated by mercury or the redistribution of contaminated water has the potential to enter the food chain through plant and livestock [3,4,5]. Once in the food chain mercury can bioaccumulate causing adverse effects to human health [6]. The exact mechanism(s) by which mercury enters the food chain remains largely unknown, and probably varies among ecosystems. Figure 1 presents multiple routes through which humans are exposed to mercury.
- Environmental mercury can exist in its elemental form, as inorganic mercury or as organic mercury. In its elemental form mercury exists as liquid metal, which in spite of its low vapor pressure (2 µm Hg), can be converted to a vapor at room temperature due to its low latent heat of evaporation (295 kJ/kg) and its relative absence from ambient air. Current sources of human exposure to elemental mercury included dental amalgam, thermometers, sphygmomanometer, barometers, fossil fuel emissions, incandescent lights, batteries, ritualistic practices using mercury, and the incineration of medical waste [7]. Toxic vapors formed from mercury vaporization or the burning of mercury containing materials can enter the respiratory system and pass readily into the circulation. The average whole body biological half-life of inhaled mercury is approximately 60 days [8]. Because mercury vapor can become lipid soluble once oxidized the potential exist for bioaccumulation in the renal cortex, liver, and especially the brain. It is estimated that the half-life of mercury in the brain can be as long as 20 years [9].
INTRODUCTION
- Inorganic mercury exists in either the mercurous and mercuric form. Like oxidized elemental mercury, mercuric salts are more water soluble and toxic than elemental mercury. Mercuric salts are also easily absorbed by the gastrointestinal tract [10]. The average whole body half-life of inorganic mercury is about 40 days [11].
- The most common form of organic mercury is methylmercury (MeHg), which is the major source of organic mercury found in the ecosystems [12]. MeHg is readily transported by water into the aquatic ecosystems. Because of its low water solubility it is considered to be relatively lipid soluble. MeHg is easily taken up by lower organisms, tends to work its way up the food chain and exhibits a proclivity to bioaccumulate in fish [13]. Fish appear to be the primary source of MeHg poisoning in humans. Through mechanisms which are not yet known, various species of fish tend to have higher rates of MeHg bioaccumulation (Table 1) [14]. The gastrointestinal tract absorbs approximately ninety five percent of ingested MeHg where it can then enter the red blood cells and the brain by binding covalently to glutathione and cysteine protein groups [15,16]. Because urinary excretion of MeHg is negligible, MeHg is primarily eliminated from the body in an inorganic form through the action of the biliary system at the rate of 1% of the body burden per day. The biological half-life of MeHg is 39 to 70 days depending on body burden. Potential sources of organic mercury included exposure to fossil fuel emissions, the incineration of medical waste, dental amalgam, and various commercial products including skin creams, germicidal soaps, various medications, teething powders, analgesics, diaper treatments, vaccinations, thermometers, sphygmomanometer, barometers, incandescent lights, and batteries [5,7]. Other sources for organic mercury include phenyl mercury compounds and ethyl mercury compounds, which were components of latex paints that were used before 1990s [12] and thimerosal which has been used as a preservative in vaccines [7]. Among the most dangerous mercury compound is dimethylmercury ((CH3)2Hg) which is toxic enough to cause death if only a few microliters is spilled on the skin, or even latex gloves [17]. Mercury poisoning can result in death, mental retardation, dysarthria, blindness, neurological deficits, loss of hearing, developmental defects, and abnormal muscle tone [7]. Table 2 presents a helpful mnemonic that practitioners can use when examining possible MeHg toxicity.
STATES OF MERCURY
- Mercury exposure has been associated with the induction of over 250 symptoms which can complicate accurate diagnosis. Differential diagnosis begins with a patient history and physical examination consistent with mercury exposure. Laboratory testing typically includes 1) blood analysis; 2) urinalysis, with a 24-hour urine analysis, and a urine challenge test with a "chelating" agent; 3) hair analysis; and (d) tissue biopsy if warranted [10,18]. Because mercury can be quickly removed from the blood, redistributed and sequestered into different tissues it is important to note that there may not be a direct correlation between blood mercury concentration and the severity of mercury poisoning. Indeed, it is thought that shortly after entering the body that mercury quickly becomes tightly bound in the brain, spinal cord, ganglia, autonomic ganglia, and peripheral motor neurons. Nonetheless although the nervous system is the primary repository for mercury exposure, the transient and residual systemic distribution of mercury has the potential to cause symptoms in a number of different organ systems. In addition reports indicate that individual genetic background may play a role in mercury toxicokinetics [19].
SYSTEMIC TOXICOLOGICAL EFFECTS OF MERCURY
- At the cellular level mercury exposure is associated with alterations in membrane permeability, changes in macromolecular structure due to its affinity for sulfhydryl and thiol groups, and DNA damage [20,21,22]. Mercury has also been shown to induce oxidative stress and mitochondrial dysfunction [23] which can result in alterations in calcium homeostasis and increased lipid peroxidation [24]. In addition, mercury may also increase radical oxygen species levels because of its ability to act as a catalyst for Fenton-type reactions [24].
CELLULAR EFFECTS OF MERCURY
- Mercury accumulation in the heart is thought to contribute to cardiomyopathy. Indeed, mercury levels in the heart tissue of individuals who died from idiopathic dilated cardiomyopathy were found to be on average 22 000 times higher than in individuals who died of other forms of heart disease [25,26]. Mercury poisoning may also cause chest pain or angina, especially in individuals under age 45 [26]. In vitro studies have indicated that MeHg can inhibit the cardioprotective activity of paraoxonase 1 [27]. There is also good evidence linking mercury with anemia including hemolytic anemia and aplastic anemia as mercury is thought to compete with iron for binding to hemoglobin which can result in impaired hemoglobin formation [28]. In addition to anemia, additional data has also suggested that mercury may be a causative factor in mononucleosis and involved in leukemia, and Hodgkin's disease [29,30,31].
- Toxic vapors formed from mercury vaporization or the burning of mercury containing materials can enter the respiratory system and pass readily into the circulation. Case control studies have demonstrated that the chronic inhalation of even low concentrations of mercury (0.7 to 42 µg/m3) can produce tremors, sleep disturbances, and impaired cognitive skills in workers [12,32,33]. Mercury poisoning is associated with several different pulmonary conditions including Young's syndrome [34], bronchitis and pulmonary fibrosis [35,36].
CARDIOVASCULAR, HEMATOLOGICAL, AND PULMONARY EFFECTS
- Mercury is absorbed through the epithelial cells when ingested. This absorbed mercury can cause various digestive disturbances as it can inhibit the production of the digestive trypsin, chymotrypsin, and pepsin along with the function of xanthine oxidase and dipeptyl peptidase IV [37]. The effects of mercury on the gastrointestinal system typically present as abdominal pain, indigestion, inflammatory bowel disease, ulcers and bloody diarrhea. Mercury ingestion has also been associated with the destruction of intestinal flora which can increase the amount of undigested food products in the blood stream causing immune mediated reactions and reduced resistance to pathogenic infection [38].
- Mercury can cause kidney damage and evidence suggests a linkage between mercury exposure and acute tubular necrosis, glomerulonephritis, chronic renal disease, renal cancer and nephrotic syndrome [35,39,40,41]. Various reports have shown mercury exposure can lead to various kidney injuries including: subacute-onset nephrotic syndrome, tubular dysfunction, secondary focal segmental glomerulosclerosis, syncreticatic nephrotic syndrome, nephritic syndrome, nephrotic-range proteinuria, glomerular disease, and membranous glomerulonephritis [42].
EFFECTS ON THE DIGESTIVE AND RENAL SYSTEMS
- Klinghardt's axiom states that "Most, if not all, chronic infectious diseases are not caused by a failure of the immune system, but are a conscious adaptation of the immune system to an otherwise lethal heavy metal environment". It has been known for many years that mercury impairs immune system function most likely via its deleterious effects on the polymorphonuclear leukocytes (PMNs). Mercury through suppression of adrenocorticosteroids production prevents normal stimulation of PMNs production and also affects PMN function by inhibiting their ability to destroy foreign substances [43]. Mercury-sensitive individuals are more likely to have allergies, asthma, and autoimmune-like symptoms, especially rheumatoid-like ones. Mercury can produce an immune response in the central nervous system, induce alterations in immune cell production and function, and modulate the production of interferon gamma and interleukin-2 [44]. With impairment comes a chronically susceptible to infections, if not chronic sickness.
- Interestingly, the ingestion of mercury is oftentimes associated with increased levels of yeasts, bacteria, and molds which are thought to function in a protective manner to absorb excess mercury from the body. Indiscriminant and rapid destruction of the Candida albicans and other pathogens by antibiotics in adults with a significant body burden of toxic metals, including mercury, may cause the sudden release of large amounts of toxic metals contained within them and be potentially very dangerous. Mercury body burden has also been associated with or implicated in a number of immune or autoimmune conditions including allergic disease, amyotrophic lateral sclerosis, arthritis, autoimmune thyroiditis, autism/attention deficit hyperactivity disorder, eczema, epilepsy, psoriasis, multiple sclerosis, rheumatoid arthritis, schizophrenia, scleroderma, and systemic lupus erythematosus [45,46,47,48,49,50,51].
EFFECTS ON THE IMMUNE SYSTEM
- It is clear that mercury is accumulated in nervous tissues all through the body [52]. The most devastating effect of mercury in the nervous system is interference with the production of energy which can impair cellular detoxification processes causing the cell to either die or live in a state of chronic malnutrition. It is thought that mercury causes neuronal problems through blockage of the P-450 enzymatic process [26]. Mercury is associated with increased tissue oxidative damage, and children with autism had significantly higher urinary levels of lipid peroxidation when compared to controls. In the peripheral nervous system, circulating inorganic mercury can be taken up into the nerve terminals where it can impair the synthesis of tubulin and actin which are important constituents of neuronal cell structure and detoxification processes [53]. Primary sensory neuropathy is a hallmark of MeHg poisoning.
- In the central nervous system mercury can damage the blood brain barrier and it facilitates penetration of the brain by other toxic metals and substances. The effects of mercury poisoning effects in the central nervous system include depression, paranoia, extreme irritability, hallucinations, an inability to concentrate, memory loss, tremors of the hands, head, lips, tongue, jaw and eyelids, weight loss, perpetually low body temperature, drowsiness, headaches, insomnia, and fatigue. Along with nervous system effects, mercury has also shown to have various effects on other special sensory systems including blindness, retinopathy, optic neuropathy, hearing loss, a reduced sense of smell, and abnormal touch sensation [54]. Autism is a syndrome characterized by impairments in social relatedness, language and communication, a need for routine and sameness, abnormal movements, and sensory dysfunction [55]. Mercury can cause immune, sensory, neurological, motor, and behavioral dysfunctions similar to traits defining or associated with autism [56] leading some to suggest that many cases of autism may be a form of mercury poisoning [55].
EFFECTS ON THE NERVOUS SYSTEM
- Low exposure levels of mercury may affect the endocrine system in animals and people by disruption of the pituitary, thyroid, adrenal glands and pancreas [57]. It is thought that mercury might impair endocrine function through its ability to reduce hormone-receptor binding or through the inhibition of one or more key enzymes or steps in hormone biosynthesis as is seen in the case of adrenal steroid biosynthesis and the inhibition of 21α-hydroxylase [58]. Hormones that appear to be the most affected by mercury are insulin, estrogen, testosterone, and adrenaline.
- Mercury can also inhibit catecholamine degradation through inactivation of S-adenosyl-methionine which can cause the accumulation of epinephrine and hyperhidrosis, tachycardia, ptyalism (hyper salivation) and hypertension [1]. In the adrenal cortex, mercury exposure has been found to be associated with lowered plasma levels of corticosterone [58]. Reduced cortisol production causes a compensatory rise in adrenocorticotropic hormone leading to adrenal hyperplasia. Mercury-induced adrenal hyperplasia may eventually stress the adrenal to a point at which there is adrenal atrophy and may be a causative factor in the development of Addison's disease [43].
- Autopsy studies in 1975 revealed that the thyroid and pituitary retain and accumulate more inorganic mercury than the kidneys [59]. Mercury levels in the pituitary gland ranged from 6.3 to 77 ppb in one study, while another found the mean levels to be 28 ppb, levels found to be neurotoxic and cytotoxic [60]. Low levels of pituitary function are associated with depression and suicidal thoughts, and appear to be a major factor in suicide of teenagers and other vulnerable groups. Because of its effect on the pituitary, mercury is known to cause frequent urination as well as high blood pressure [61].
- The thyroid is one of the largest endocrine glands in the body. The thyroid controls how quickly the body burns energy, makes proteins, and how sensitive the body should be to other hormones. Like the pituitary, the thyroid displays an affinity for accumulating mercury. Mercury blocks thyroid hormone production by occupying iodine-binding sites and inhibiting or altering hormone action leading to the impairment of body temperature control, hypothyroidism, thyroid inflammation and depression [43,61].
- Like the thyroid, the pancreas is also susceptible to the toxic effects of mercury. Insulin, the molecule involved in diabetes, has three sulfur-binding sites which can be bound by mercury causing the interference with normal biological function and a dysregulation of blood glucose levels [62].
EFFECTS ON THE ENDOCRINE SYSTEM
- Mercury can precipitate pathophysiological changes along the hypothalamus-pituitary-adrenal and gonadal axis that may affect reproductive function by altering the circulating of levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), inhibin, estrogen, progesterone, and the androgens [63,64]. Reduced fertility among dental assistants with occupational exposure to mercury has been noted [65,66]. Studies in Hong Kong demonstrated that increased mercury levels were associated with infertility in both men and women [67]. In males, mercury can have adverse effects on spermatogenesis [68], epididymal sperm count, and testicular weight. Evidence also exists linking mercury with erectile dysfunction [64]. In females, mercury has been shown to inhibit the release of FSH and LH from the anterior pituitary which in turn can effect estrogen and progesterone levels leading to ovarial dysfunction, painful or irregular menstruation, premature menopause, and tipped uterus [62]. There is good evidence linking mercury with menstrual disorders including abnormal bleeding, short, long, irregular cycles, and painful periods [63].
EFFECTS ON THE REPRODUCTIVE SYSTEM
- In addition to reproductive issues, mercury is also associated with the fetotoxicity which can present as miscarriage, spontaneous abortions, stillbirth, and low birth weights [69]. In the neonate, mercury exposure during pregnancy has been linked to neural tube defects, craniofacial malformations, delayed growth, and others [69]. Mercury is known to cross the placenta where it can inhibit fetal brain development resulting in cerebral palsy and psychomotor retardation in the latter stages of development [70,71]. In primates maternal MeHg blood levels were moderatelyrelated to increased abortion rates and decreased oregnancy rates [72]. Embryopathic effects of MeHg in humans have also been reported. Fetal autopsies indicated a generalized hypoplasia of the cerebellum, decreased number of nerve cells in the cerebral cortex, marked decrease in total brain weight, abnormal neuron migration, and brain centers and layer deranged organization [73,74,75,76]. MeHg easily enters through the placenta and damages the brain of the fetus. Many exposed feti go on to develop infantile cerebral palsy and there may be a relation with the development of Minamata disease. Babies may be born with a variety of birth defects. A study of 64 children exposed in utero to mercury and showing mercury associated damage included the following signs and symptoms: mental retardation (100%), primitive reflexes (100%), strabismus (77%), cerebellar ataxia (100%), dysarthria (100%), chorea and athetosis (95%), deformed limbs (100%), hyper salivation (95%), epileptic attacks (82%), and growth disorders (100%) [6]. Mercury inhibits the trans membrane transport of nutrients including selenium in the placenta. In animal experiments it has also been shown that there is a much higher accumulation of mercury in the fetal brain tissue than in the maternal brain tissue [77].
FETOTOXICITY
- It is evident by the number of organ systems and cellular functions affected by mercury that exposure to the various form of mercury is detrimental to public health. Evaluation of the epidemiological consequences of mercury toxicity over the years has added greatly to the understanding of mercury toxicity and its human impact. History has left us with a wide array of information regarding the effects of mercury toxicity: the earliest recorded death by mercury of the Qin Shi Huang first emperor to unify China [78], the "Mad Hatter disease" among milliners in the 18th and 19th centuries [79], the mercury spill on board the two British ships the Her Majesty's ship (HMS) Triumph and HMS Philpps in 1810 [80,81], the apparent death of approximately 60 men during the construction of Saint Isaac's Cathedral in Russia between 1818 to 1858 from the gold amalgam used for gilding [82], the mysterious death of actress Olive Thomas in 1920 from ingestion of her husband's mercury pill used at the time to treat syphilis [83,84], the event at the Norwich England seed packing facility in the 1930s where the term "Hunter-Russell syndrome" originates [85], the 1950s industrial spill in Minamata and Niigat Japan where it was defined as "Minamata disease" [4], the rural poisoning in Iraq in 1971 to 1972 from MeHg-based fungicide [86], Karen Wetterhahn's death at Dartmouth College in 1996 from a drop of dimethymercury of her latex gloves [87], Tony Winneet's accidental death from using liquid mercury to extract gold from old computer parts [88], the current finding of mercury in 6.0% of skin-lightening products tested in one study [89] and 47% of products tested in a Somali community contained mercury [90], the long term effects of the California Gold mining impact on mercury redistribution and potential impact on human health [91], and the numerous links to human consumption of mercury laden fish [92,93]. All of these events have left us with an indelible account of the detrimental effects of mercury on human health. In light of these historic events and the toxicological evidence presenting in this review regarding the systemic effects of mercury on cellular, cardiovascular, hematological, pulmonary, renal, immunological, neurological, endocrine, reproductive, and embryonic development, efforts should be made to insure adequate steps are taken in public health and prevention to reduce the occurrence of mercury exposure and raise public awareness.
CONCLUSION
- 1. Clifton JC 2nd. Mercury exposure and public health. Pediatr Clin North Am 2007;54(2):237-269. 17448359ArticlePubMed
- 2. US Department of Health and Human Services, Public Health Service. Toxicological profile for mercury. Atlanta: US Department of Health and Human Services; 1999. p. 1-600
- 3. Rice GE, Ambrose RB Jr, Bullock OR Jr, Smawtout J. Mercury study report to Congress. Durham: US Environmental Protection Agency; 1997. p. 1.1-6.30
- 4. Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics 2004;113(4 Suppl):1023-1029. 15060195ArticlePubMedPDF
- 5. Goldman LR, Shannon MW. American Academy of Pediatrics: Committee on Environmental Health. Technical report: mercury in the environment: implications for pediatricians. Pediatrics 2001;108(1):197-205. 11433078ArticlePubMed
- 6. Harada M, Nakachi S, Cheu T, Hamada H, Ono Y, Tsuda T, et al. Monitoring of mercury pollution in Tanzania: relation between head hair mercury and health. Sci Total Environ 1999;227(2-3):249-256. 10231987ArticlePubMed
- 7. Guzzi G, La Porta CA. Molecular mechanisms triggered by mercury. Toxicology 2008;244(1):1-12. 18077077ArticlePubMed
- 8. Chang LW. Neurotoxic effects of mercury: a review. Environ Res 1977;14(3):329-373. 338298ArticlePubMed
- 9. Friberg L, Mottet NK. Accumulation of methylmercury and inorganic mercury in the brain. Biol Trace Elem Res 1989;21: 201-206. 2484587ArticlePubMed
- 10. Magos L, Clarkson TW. Overview of the clinical toxicity of mercury. Ann Clin Biochem 2006;43(Pt 4):257-268. 16824275ArticlePubMed
- 11. Clarkson TW. Mercury: major issues in environmental health. Environ Health Perspect 1993;100: 31-38. 8354179ArticlePubMedPMC
- 12. Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 2006;36(8):609-662. 16973445ArticlePubMed
- 13. Mahaffey KR. Methylmercury: a new look at the risks. Public Health Rep 1999;114(5):396-399. 10590759PubMedPMC
- 14. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296(15):1885-1899. 17047219ArticlePubMed
- 15. Sarafian T, Verity MA. Oxidative mechanisms underlying methyl mercury neurotoxicity. Int J Dev Neurosci 1991;9(2):147-153. 1905456ArticlePubMed
- 16. Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury. 1999. cited 2014 Mar 21. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=115&tid=24
- 17. Joshi D, Mittal DK, Shukla S, Srivastav AK. Therapeutic potential of N-acetyl cysteine with antioxidants (Zn and Se) supplementation against dimethylmercury toxicity in male albino rats. Exp Toxicol Pathol 2012;64(1-2):103-108. 20688495ArticlePubMed
- 18. Schoeman K, Bend JR, Koren G. Hair methylmercury: a new indication for therapeutic monitoring. Ther Drug Monit 2010;32(3):289-293. 20445486ArticlePubMed
- 19. Gundacker C, Gencik M, Hengstschlager M. The relevance of the individual genetic background for the toxicokinetics of two significant neurodevelopmental toxicants: mercury and lead. Mutat Res 2010;705(2):130-140. 20601101ArticlePubMed
- 20. Naganuma A, Furuchi T, Miura N, Hwang GW, Kuge S. Investigation of intracellular factors involved in methylmercury toxicity. Tohoku J Exp Med 2002;196(2):65-70. 12498317ArticlePubMed
- 21. Wang L, Jia G. Progress in developmental toxicity of methylmercury. Wei Sheng Yan Jiu 2005;34(5):633-635. 16329617PubMed
- 22. Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 2008;128(4):501-523. 19106443PubMed
- 23. Lund BO, Miller DM, Woods JS. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem Pharmacol 1993;45(10):2017-2024. 8512585ArticlePubMed
- 24. Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. Effects of micronutrients on metal toxicity. Environ Health Perspect 1998;106(Suppl 1):203-216. 9539014Article
- 25. Haffner HT, Erdelkamp J, Goller E, Schweinsberg F, Schmidt V. Morphological and toxicological findings after intravenous injection of metallic mercury. Dtsch Med Wochenschr 1991;116(36):1342-1346. 1884673ArticlePubMed
- 26. Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, et al. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J Am Coll Cardiol 1999;33(6):1578-1583. 10334427ArticlePubMed
- 27. Drescher O, Dewailly E, Diorio C, Ouellet N, Sidi EA, Abdous B, et al. Methylmercury exposure, PON1 gene variants and serum paraoxonase activity in Eastern James Bay Cree adults. J Expo Sci Environ Epidemiol 2014. http://dx.doi.org/10.1038/jes.2013.96Article
- 28. Pyszel A, Wrobel T, Szuba A, Andrzejak R. Effect of metals, benzene, pesticides and ethylene oxide on the haematopoietic system. Med Pr 2005;56(3):249-255. 16218139PubMed
- 29. Kinjo Y, Akiba S, Yamaguchi N, Mizuno S, Watanabe S, Wakamiya J, et al. Cancer mortality in Minamata disease patients exposed to methylmercury through fish diet. J Epidemiol 1996;6(3):134-138. 8952217ArticlePubMed
- 30. Robinson MM. Dermatitis medicamentosa simulating Hodgkin's disease due to mercury compounds. Ann Allergy 1952;10(1):21-23. 14894984PubMed
- 31. Flanders RA. Mercury in dental amalgam: a public health concern? J Public Health Dent 1992;52(5):303-311. 1404077ArticlePubMed
- 32. Liang YX, Sun RK, Sun Y, Chen ZQ, Li LH. Psychological effects of low exposure to mercury vapor: application of a computer-administered neurobehavioral evaluation system. Environ Res 1993;60(2):320-327. 8472661ArticlePubMed
- 33. Heyer NJ, Echeverria D, Bittner AC Jr, Farin FM, Garabedian CC, Woods JS. Chronic low-level mercury exposure, BDNF polymorphism, and associations with self-reported symptoms and mood. Toxicol Sci 2004;81(2):354-363. 15254338ArticlePubMed
- 34. Hendry WF, A'Hern RP, Cole PJ. Was Young's syndrome caused by exposure to mercury in childhood? BMJ 1993;307(6919):1579-1582. 8292944ArticlePubMedPMC
- 35. Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 2003;18(3):149-175. 12740802ArticlePubMed
- 36. Haddad JK, Stenberg E Jr. Bronchitis due to acute mercury inhalation. Report of two cases. Am Rev Respir Dis 1963;88: 543-545. 14068440PubMed
- 37. Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol 2003;16(3):189-199. 14611720ArticlePubMedPDF
- 38. Summers AO, Wireman J, Vimy MJ, Lorscheider FL, Marshall B, Levy SB, et al. Mercury released from dental "silver" fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother 1993;37(4):825-834. 8280208ArticlePubMedPMC
- 39. Li SJ, Zhang SH, Chen HP, Zeng CH, Zheng CX, Li LS, et al. Mercury-induced membranous nephropathy: clinical and pathological features. Clin J Am Soc Nephrol 2010;5(3):439-444. 20089494ArticlePubMedPMC
- 40. Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health 2012;45(6):344-352. 23230464ArticlePubMedPMCPDF
- 41. Oliveira DB, Foster G, Savill J, Syme PD, Taylor A. Membranous nephropathy caused by mercury-containing skin lightening cream. Postgrad Med J 1987;63(738):303-304. 3684841ArticlePubMedPMC
- 42. Miller S, Pallan S, Gangji AS, Lukic D, Clase CM. Mercury-associated nephrotic syndrome: a case report and systematic review of the literature. Am J Kidney Dis 2013;62(1):135-138. 23602193ArticlePubMed
- 43. Wada H, Cristol DA, McNabb FM, Hopkins WA. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol 2009;43(15):6031-6038. 19731714ArticlePubMed
- 44. Shenker BJ, Rooney C, Vitale L, Shapiro IM. Immunotoxic effects of mercuric compounds on human lymphocytes and monocytes. I. Suppression of T-cell activation. Immunopharmacol Immunotoxicol 1992;14(3):539-553. 1517533ArticlePubMed
- 45. Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett 2010;198(2):182-190. 20600710ArticlePubMedPMC
- 46. Warren HV. Geology, trace elements and health. Soc Sci Med 1989;29(8):923-926. 2683120ArticlePubMed
- 47. Singh VK. Phenotypic expression of autoimmune autistic disorder (AAD): a major subset of autism. Ann Clin Psychiatry 2009;21(3):148-161. 19758536PubMed
- 48. Schofield P. Dementia associated with toxic causes and autoimmune disease. Int Psychogeriatr 2005;17(Suppl 1):S129-S147. 16240488ArticlePubMed
- 49. Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology 2009;30(5):761-765. 19632272ArticlePubMedPMC
- 50. Hybenova M, Hrda P, Prochazkova J, Stejskal V, Sterzl I. The role of environmental factors in autoimmune thyroiditis. Neuro Endocrinol Lett 2010;31(3):283-289. 20588228PubMed
- 51. Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 2010;22(2):219-225. 20087185ArticlePubMed
- 52. Ceccatelli S, Dare E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact 2010;188(2):301-308. 20399200ArticlePubMed
- 53. Kazantzis G. Mercury exposure and early effects: an overview. Med Lav 2002;93(3):139-147. 12197264PubMed
- 54. Wu MF, Ison JR, Wecker JR, Lapham LW. Cutaneous and auditory function in rats following methyl mercury poisoning. Toxicol Appl Pharmacol 1985;79(3):377-388. 4035685ArticlePubMed
- 55. Solt I, Bornstein J. Childhood vaccines and autism: much ado about nothing? Harefuah 2010;149(4):251-255. 20812501PubMed
- 56. Bhardwaj A, Kar JP, Thakur OP, Srivastava P, Sehgal HK. Electrical characteristics of PbSe nanoparticle/Si heterojunctions. J Nanosci Nanotechnol 2009;9(10):5953-5957. 19908480ArticlePubMed
- 57. Minoia C, Ronchi A, Pigatto P, Guzzi G. Effects of mercury on the endocrine system. Crit Rev Toxicol 2009;39(6):538. 19545200ArticlePubMed
- 58. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 2009;12(3):206-223. 19466673ArticlePubMed
- 59. Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol 2009;39(3):228-269. 19280433ArticlePubMed
- 60. Nylander M, Weiner J. Mercury and selenium concentrations and their interrelations in organs from dental staff and the general population. Br J Ind Med 1991;48(11):729-734. 1835404ArticlePubMedPMC
- 61. McGregor AJ, Mason HJ. Occupational mercury vapour exposure and testicular, pituitary and thyroid endocrine function. Hum Exp Toxicol 1991;10(3):199-203. 1678950ArticlePubMed
- 62. Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang CY, et al. Methylmercury induces pancreatic beta-cell apoptosis and dysfunction. Chem Res Toxicol 2006;19(8):1080-1085. 16918248ArticlePubMed
- 63. Davis BJ, Price HC, O'Connor RW, Fernando R, Rowland AS, Morgan DL. Mercury vapor and female reproductive toxicity. Toxicol Sci 2001;59(2):291-296. 11158722ArticlePubMed
- 64. Schrag SD, Dixon RL. Occupational exposures associated with male reproductive dysfunction. Annu Rev Pharmacol Toxicol 1985;25: 567-592. 2408559ArticlePubMed
- 65. Rowland AS, Baird DD, Weinberg CR, Shore DL, Shy CM, Wilcox AJ. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occup Environ Med 1994;51(1):28-34. 8124459ArticlePubMedPMC
- 66. Colquitt PJ. The effect of occupational exposure to mercury vapour on the fertility of female dental assistants. Occup Environ Med 1995;52(3):214. 7735398ArticlePubMedPMC
- 67. Dickman MD, Leung CK, Leong MK. Hong Kong male subfertility links to mercury in human hair and fish. Sci Total Environ 1998;214: 165-174. 9646524ArticlePubMed
- 68. Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, et al. Testicular toxicity in mercuric chloride treated rats: association with oxidative stress. Reprod Toxicol 2009;28(1):81-89. 19427169ArticlePubMed
- 69. Yoshida M. Placental to fetal transfer of mercury and fetotoxicity. Tohoku J Exp Med 2002;196(2):79-88. 12498319ArticlePubMed
- 70. Castoldi AF, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull 2001;55(2):197-203. 11470315ArticlePubMed
- 71. Myers GJ, Davidson PW. Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect 1998;106(Suppl 3):841-847. 9646047Article
- 72. Burbacher TM, Monnett C, Grant KS, Mottet NK. Methylmercury exposure and reproductive dysfunction in the nonhuman primate. Toxicol Appl Pharmacol 1984;75(1):18-24. 6464019ArticlePubMed
- 73. Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: a major effect of methylmercury poisoning in utero. J Neuropathol Exp Neurol 1978;37(6):719-733. 739273ArticlePubMed
- 74. Grandjean P, Weihe P, Nielsen JB. Methylmercury: significance of intrauterine and postnatal exposures. Clin Chem 1994;40(7 Pt 2):1395-1400. 8013126ArticlePubMedPDF
- 75. Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, et al. Methylmercury poisoning in Iraq. Science 1973;181(4096):230-241. 4719063ArticlePubMed
- 76. Mottet NK, Shaw CM, Burbacher TM. Health risks from increases in methylmercury exposure. Environ Health Perspect 1985;63: 133-140. 3908085ArticlePubMedPMC
- 77. Meacham CA, Freudenrich TM, Anderson WL, Sui L, Lyons-Darden T, Barone S Jr, et al. Accumulation of methylmercury or polychlorinated biphenyls in in vitro models of rat neuronal tissue. Toxicol Appl Pharmacol 2005;205(2):177-187. 15893545ArticlePubMed
- 78. Zhao HL, Zhu X, Sui Y. The short-lived Chinese emperors. J Am Geriatr Soc 2006;54(8):1295-1296. 16914004ArticlePubMed
- 79. Waldron HA. Did the Mad Hatter have mercury poisoning? Br Med J (Clin Res Ed) 1983;287(6409):1961ArticlePubMedPMC
- 80. Doherty MJ. The quicksilver prize: mercury vapor poisoning aboard HMS Triumph and HMS Phipps. Neurology 2004;62(6):963-966. 15037700ArticlePubMed
- 81. Burnett W. An account of the effect of mercurial vapours on the crew of his majesty's ship triumph, in the year 1810. Philos Trans R Soc Lond 1823;113: 402-408Article
- 82. Westbrook JH. Metallurgical and chemical applications of intermetallics. MRS Bull 1996;21(5):37-43Article
- 83. Broussard LA, Hammett-Stabler CA, Winecker RE, Ropero-Miller JD. The toxicology of mercury. Lab Med 2002;33(8):614-625Article
- 84. Hyman HT, Chargin L, Leifer W. Massive dose arsenotherapy of syphilis by the intravenous drip method: five-year observations. Am J Med Sci 1939;197(4):480-484Article
- 85. Hunter D, Bomford RR, Russell DS. Poisoning by methylmercury compounds. Q J Med 1940;9(3):193-226
- 86. Engler R. Technology out of control. Nation 1985;240(16):488
- 87. Witt SF. OSHA safety hazard information bulletin on dimethylmercury. Washington, DC: US Department of Labor, Occupational Safety and Health Administration; 1991. p. 1
- 88. Tulsa World. Colbert man dies from mercury poisoning. 2008. 4. 01. cited 2014 Mar 21. Available from: http://archive.is/kOyZ
- 89. Hamann CR, Boonchai W, Wen L, Sakanashi EN, Chu CY, Hamann K, et al. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol 2014;70(2):281-287. 24321702ArticlePubMed
- 90. Adawe A, Oberg C. Skin-lightening practices and mercury exposure in the Somali community. Minn Med 2013;96(7):48-49. 24133891
- 91. Singer MB, Aalto R, James LA, Kilham NE, Higson JL, Ghoshal S. Enduring legacy of a toxic fan via episodic redistribution of California gold mining debris. Proc Natl Acad Sci U S A 2013;110(46):18436-18441. 24167273ArticlePubMedPMC
- 92. Nunes E, Cavaco A, Carvalho C. Children's health risk and benefits of fish consumption: risk indices based on a diet diary follow-up of two weeks. J Toxicol Environ Health A 2014;77(1-3):103-114. 24555651ArticlePubMed
- 93. Rodríguez Martín-Doimeadios RC, Berzas Nevado JJ, Guzman Bernardo FJ, Jimenez Moreno M, Arrifano GP, Herculano AM, et al. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ Sci Pollut Res Int 2014. http://dx.doi.org/10.1007/s11356-014-2680-7Article
REFERENCES
| Species | Mercury content (ppm) | Safety |
|---|---|---|
| Anchovies | 0.0431 | Eco-good |
| Butterfish | 0.0581 | Eco-good |
| Catfish | 0.049±0.084 | Eco-good |
| Crab (blue, king, and snow) | 0.060±0.112 | Eco-good |
| Crawfish | 0.033±0.012 | Eco-good |
| Flatfish (flounder, plaice, and sole) | 0.045±0.049 | Eco-good |
| Haddock | 0.031±0.021 | Eco-good |
| Herring | 0.0441 | Eco-good |
| Mackerel, Atlantic | 0.0501 | Eco-good |
| Mullet | 0.0461 | Eco-good |
| Oysters (farmed) | 0.013±0.042 | Eco-good |
| Pollock | 0.041±0.106 | Eco-good |
| Salmon, wild (Alaska) | 0.014±0.041 | Eco-good |
| Sardines, Pacific (US) | 0.016±0.007 | Eco-good |
| Scallops | 0.0501 | Eco-good |
| Squid | 0.0701 | Eco-good |
| Tilapia | 0.0101 | Eco-good |
| Trout, rainbow (farmed, freshwater) | 0.072±0.143 | Eco-good |
| Tuna, albacore (US, Canada) | 0.353±0.126 | Eco-bad |
| Atlantic cod (also known as gadus morhua, rock cod, codling, scrod cod) | 0.095±0.080 | Eco-bad |
| Bigeye/yellowfin tuna (imported long- line) | 0.325±0.220 | Eco-bad |
| Bluefish | 0.337±0.127 | Eco-bad |
| Chilean sea bass | 0.386±0.384 | Eco-bad |
| Carp | 0.1401 | Eco-bad |
| Grouper | 0.465±0.293 | Eco-bad |
| Halibut | 0.252±0.233 | Eco-bad |
| Imported swordfish | 0.976±0.510 | Eco-bad |
| King mackerel | 0.7301 | Eco-bad |
| Marlin | 0.485±0.237 | Eco-bad |
| Monkfish | 0.1801 | Eco-bad |
| Orange roughy | 0.554±0.148 | Eco-bad |
| Sablefish (Alaska, Canada) | 0.2201 | Eco-bad |
| Shark (Carcarhinus limbatus) or short- fin mako (Isurus oxyrinchus) | 0.988±0.631 | Eco-bad |
| Snapper | 0.189±0.274 | Eco-bad |
| Tilefish (golden bass, Atlantic) | 1.450±0.122 | Eco-bad |
Figure & Data
References
Citations

- Biomaterial-Driven Novel Cost-effective Photonic Sensor for Trace Determination of Lead in Aqueous Solutions
Rajib Biswas, Rajon Bhuyan
IETE Journal of Research.2024; 70(1): 230. CrossRef - Interface modulation of Mn-N4-C with optimized oxygen-containing functional groups for highly efficient mercury adsorption
Jisai Chen, Zhijie Huang, Yongxian Zhou, Jiaxing Li, Sun Hu, Wenjun Huang, Zan Qu
Journal of Hazardous Materials.2024; 461: 132498. CrossRef - Health risk assessment of lead‐zinc mine tailing in Angoran, Zanjan, Iran
Ahmad Akhavan, Ahmad Golchin
Environmental Quality Management.2024; 33(3): 141. CrossRef - Colorimetric detection of fluoride and mercury (II) ions using isatin Schiff base skeleton bearing pyridine-2-carboxamidine moiety: Experimental and theoretical studies
Parinaz Eshghi, Leila Moafi, Mohammad Alidoosti, Davoud Nasr Esfahani
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2024; 305: 123467. CrossRef - The GSTP1 rs1695 Polymorphism Is Associated with Mercury Levels and Neurodevelopmental Delay in Indigenous Munduruku Children from the Brazilian Amazon
Mayara Calixto da Silva, Paulo Cesar Basta, Cristina Barroso Hofer, Mirian Akiko Furutani de Oliveira, Joeseph William Kempton, Rogério Adas Ayres de Oliveira, Ana Claudia Santiago de Vasconcellos, Jamila Alessandra Perini
Toxics.2024; 12(6): 441. CrossRef - A novel reaction-based ratiometric fluorescent probe with large Stokes shift for the detection of Hg2+ in river sample
Aishan Ren, Wenqin Yao, Wei Xie, Dongjian Zhu
Journal of Molecular Structure.2024; 1298: 137035. CrossRef - Structural-property relationship in poly(vinyl aniline-co-maleic anhydride) for effective Hg2+ ions adsorption
Yashwant Shandil, Umesh K. Dautoo, Sunita Ranote, Ghanshyam S. Chauhan
Colloids and Surfaces A: Physicochemical and Engineering Aspects.2024; 681: 132836. CrossRef - Green synthesis of elephant manure-derived carbon dots and multifunctional applications: Metal sensing, antioxidant, and rice plant promotion
Chuleekorn Seesuea, Sompong Sansenya, Pattanapong Thangsunan, Kanokorn Wechakorn
Sustainable Materials and Technologies.2024; 39: e00786. CrossRef - HgCl2 exposure mediates pyroptosis of HD11 cells and promotes M1 polarization and the release of inflammatory factors through ROS/Nrf2/NLRP3
Jiaqi Wang, Yilin Yin, Qirui Zhang, Xinrui Deng, Zhiruo Miao, Shiwen Xu
Ecotoxicology and Environmental Safety.2024; 269: 115779. CrossRef - Galinstan Liquid Metal Electrical Contacts for Monolayer-Modified Silicon Surfaces
Carlos Hurtado, Tony Andreoli, Anton P. Le Brun, Melanie MacGregor, Nadim Darwish, Simone Ciampi
Langmuir.2024; 40(1): 201. CrossRef - Poison in the nursery: Mercury contamination in the tadpole-rearing sites of an Amazonian frog
Lia Schlippe-Justicia, Jérémy Lemaire, Carolin Dittrich, Martin Mayer, Paco Bustamante, Bibiana Rojas
Science of The Total Environment.2024; 912: 169450. CrossRef - Bismuth Nanoparticles Modified Indium Tin Oxide-Coated with Polyethene Terephthalate Electrode Using Hydrothermal Method for Pb Detection
Nurul Hidayah Ramli, Noorhashimah Mohamad Nor, Liew Xian Yun, Khairunisak Abdul Razak
Applied Mechanics and Materials.2024; 918: 139. CrossRef - Blood Pb levels are associated with prostate cancer prevalence among general adult males: Linking National Cancer Registry (2002–2017) and KNHANES (2008–2017) databases of Korea
Yonju Nam, Suhyun Park, Ejin Kim, Inae Lee, Young Joo Park, Tae-Yong Kim, Min Joo Kim, Shinje Moon, Sangah Shin, Ho Kim, Kyungho Choi
International Journal of Hygiene and Environmental Health.2024; 256: 114318. CrossRef - Association of exposure to multiple heavy metals during pregnancy with the risk of gestational diabetes mellitus and insulin secretion phase after glucose stimulation
Shitao He, Tingting Jiang, Dongyang Zhang, Mengzhu Li, Tao Yu, Muxin Zhai, Bingxia He, Tao Yin, Xin Wang, Fangbiao Tao, Yuyou Yao, Dongmei Ji, Yuanyuan Yang, Chunmei Liang
Environmental Research.2024; 248: 118237. CrossRef - Association between multiple sclerosis and urinary levels of toxic metals and organophosphates: A cross-sectional study in Israel
Ayelet Armon-Omer, Tarek Mansor, Michael Edelstein, Elena Bukovetzky, Luda Groisman, Efrat Rorman, Adi Sharabi Nov, Radi Shahien
Multiple Sclerosis and Related Disorders.2024; 83: 105445. CrossRef - Assessing the impact and mechanisms of environmental pollutants (heavy metals and pesticides) on the male reproductive system: a comprehensive review
Rohit Gautam, Eepsita Priyadarshini, Arbind Kumar Patel, Taruna Arora
Journal of Environmental Science and Health, Part C.2024; 42(2): 126. CrossRef - Neurological risks arising from the bioaccumulation of heavy metal contaminants: A focus on mercury
Tianyu Dong, Hanxuan Li
Environmental Toxicology.2024; 39(5): 2692. CrossRef - On-Off-On Fluorometric Detection of Hg(II) and L-Cysteine Using Red Emissive Nitrogen-Doped Carbon Dots for Environmental and Clinical Sample Analysis
D James Nelson, N Vasimalai, S Abraham John, M G Sethuraman
Journal of Fluorescence.2024;[Epub] CrossRef - Human Health Risk Assessment from Mercury-Contaminated Soil and Water in Abu Hamad Mining Market, Sudan
Ahmed Elwaleed, HuiHo Jeong, Ali H. Abdelbagi, Nguyen Thi Quynh, Tetsuro Agusa, Yasuhiro Ishibashi, Koji Arizono
Toxics.2024; 12(2): 112. CrossRef - Elucidating the link between thyroid cancer and mercury exposure: a review and meta-analysis
Alyssa M. Webster, Dylan Pinion, Eric Pineda, Hadeel Aboueisha, Mohammad H. Hussein, Manal S. Fawzy, Eman A. Toraih, Emad Kandil
Environmental Science and Pollution Research.2024; 31(9): 12841. CrossRef - The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review
Yuan-Seng Wu, Ahmed I. Osman, Mohamed Hosny, Ahmed M. Elgarahy, Abdelazeem S. Eltaweil, David W. Rooney, Zhonghao Chen, Nur Syafiqah Rahim, Mahendran Sekar, Subash C. B. Gopinath, Nur Najihah Izzati Mat Rani, Kalaivani Batumalaie, Pow-Seng Yap
ACS Omega.2024; 9(5): 5100. CrossRef - Mercury bioaccumulation and Hepatozoon spp. infections in two syntopic watersnakes in South Carolina
M. Kyle Brown, David Lee Haskins, Melissa A. Pilgrim, Tracey D. Tuberville
Ecotoxicology.2024; 33(2): 164. CrossRef - Serum levels of inflammatory cytokines in mercury mining workers in a precarious situation: A preliminary study
Kelvin Saldaña-Villanueva, Ana K González-Palomo, Karen B Méndez-Rodríguez, Arturo Gavilán-García, Gamaliel Benítez-Arvizu, Fernando Diaz-Barriga, Luz Alcantara-Quintana, Francisco J Pérez-Vázquez
Toxicology and Industrial Health.2024; 40(3): 134. CrossRef - Critical period of exposure to mercury and the diagnostic of autism spectrum disorder: A systematic review
Bruna Bittencourt Netto, Elica Pizzolo da Silva, Maiara de Aguiar da Costa, Victória Linden de Rezende, Sofia Januário Bolan, Luciane Bisognin Ceretta, Michael Aschner, Diogo Dominguini, Cinara Ludvig Gonçalves
Journal of Neurochemistry.2024;[Epub] CrossRef - Pro-vegetarian dietary patterns and essential and heavy metal exposure in children of 4-5-years from the INfancia y medio Ambiente cohort (INMA)
Alejandro Oncina-Cánovas, Jesús Vioque, Gabriel Riutort-Mayol, Raquel Soler-Blasco, Amaia Irizar, Ziortza Barroeta, Ana Fernández-Somoano, Adonina Tardón, Martine Vrijheid, Mònica Guxens, Manus Carey, Caroline Meharg, Kathryn Ralphs, Coalain McCreanor, An
International Journal of Hygiene and Environmental Health.2024; 257: 114344. CrossRef - Could the gut microbiota be capable of making individuals more or less susceptible to environmental toxicants?
Marcella S.A. Santiago, Maria Christina W. Avellar, Juliana E. Perobelli
Toxicology.2024; 503: 153751. CrossRef - Appraisal of pollution levels and non-carcinogenic health risks associated with the emergence of heavy metals in Indonesian community water for sanitation, hygiene, and consumption
Nurul Fahimah, Indah Rachmatiah Siti Salami, Katharina Oginawati, Haryo Mubiarto
Emerging Contaminants.2024; 10(3): 100313. CrossRef - Level of Heavy Metals and Potential Ecological Risks in Irrigated Horticultural Farms in the Vicinity of Lake Ziway, Central Ethiopian Rift Valley Region
GirmaTilahun Yimer, Victor Wepener
Journal of Toxicology.2024; 2024: 1. CrossRef - A carbon dots-MnO2 nanosheet-based turn-on pseudochemodosimeter as low-cost probe for selective detection of hazardous mercury ion contaminations in water
Ankit Thakuri, Akhil A. Bhosle, Sharanabasava D. Hiremath, Mainak Banerjee, Amrita Chatterjee
Journal of Hazardous Materials.2024; 469: 133998. CrossRef - Styryl hemicyanine-DNA assembly for selective Hg2+ sensing and molecular computing
Awad I. Said, Meglena Kandinska, Aleksey Vasilev, Ivo Grabchev
Journal of Photochemistry and Photobiology A: Chemistry.2024; 452: 115590. CrossRef - Genomic investigation on genes related to mercury metabolism in Amazonian indigenous populations
Victor Hugo Valente Carvalho, Juliana Carla Gomes Rodrigues, Lui Wallacy Morikawa Souza Vinagre, Esdras Edgar Batista Pereira, Natasha Monte, Marianne Rodrigues Fernandes, André Maurício Ribeiro-dos-Santos, João Farias Guerreiro, Ândrea Ribeiro-dos-Santos
Science of The Total Environment.2024; 923: 171232. CrossRef - Review of the Influence of Climate Change on the Hydrologic Cycling and Gaseous Fluxes of Mercury in Boreal Peatlands: Implications for Restoration
Randy Kolka, Caroline Pierce, Isabella Garrioch, Kevin Behrens, Brandy M. Toner
Water.2024; 16(8): 1154. CrossRef - Mechanistic Evidence for Hg Removal from Wastewater by Biologically Produced Sulfur
Seok-Soon Jeong, Byung-Jun Park, Jung-Hwan Yoon, Mary Beth Kirkham, Jae-E. Yang, Hyuck-Soo Kim
Toxics.2024; 12(4): 278. CrossRef - Heavy Metal Exposure and Cardiovascular Disease
Ziwei Pan, Tingyu Gong, Ping Liang
Circulation Research.2024; 134(9): 1160. CrossRef - Development and sensitivity analysis of MWCNTs coated D-shaped plastic optical fiber sensor for the detection of mercury
Abdul Ali Khan, Punithavathi M. Thirunavakkarasu, Ahmad Shukri Muhammad Noor, Norazlina bte Saidin, Nawaf Waqas
Optical Fiber Technology.2024; 85: 103813. CrossRef - Mercurio total (Hg-T) en ictiofauna de mayor consumo en San Marcos - Sucre, Colombia
Daniel Esteban Romero-Suárez, Liseth Pérez-Flórez , Adolfo Consuegra-Solórzano, Jhon Vidal-Durango , Jorge Buelvas-Soto, José Marrugo-Negrete
Revista MVZ Córdoba.2024; 27(3): e2488. CrossRef - Trace Element Concentration in the Blood and Aqueous Humor of Subjects with Eye Cataract
Giovanni Forte, Edoardo Trovato Battagliola, Mariaelena Malvasi, Niccolò Ruberti, Pierluigi Daniele, Alberto Mantovani, Beatrice Bocca, Elena Pacella
Biological Trace Element Research.2024;[Epub] CrossRef - Westerlies-driven transboundary transport of atmospheric mercury to the north-central Tibetan Plateau
Shiwei Sun, Ming Ma, Junming Guo, Xiaobo He, Xiufeng Yin, Tao Sun, Qianggong Zhang, Shichang Kang
Science of The Total Environment.2024; 932: 173135. CrossRef - A comparison of Fe(III) to Fe(II) reduction methods in iron analysis via titration
Mahdi Ostadrahimi, Saeed Farrokhpay, Khodayar Karimnejad, Azam Rahimian, Mostafa Molavi, Ghobad Shahkarami
Chemical Papers.2024; 78(9): 5407. CrossRef - Assessment of macro, trace and toxic element intake from rice: differences between cultivars, pigmented and non-pigmented rice
Xingyong Liu, Qian Li, Benlin Yin, Hongmei Yan, Yunmei Wang
Scientific Reports.2024;[Epub] CrossRef - Prevention of Parkinson’s Disease: From Risk Factors to Early
Interventions
Ming Guan Ng, Brendan Jun Lam Chan, Rhun Yian Koh, Khuen Yen Ng, Soi Moi Chye
CNS & Neurological Disorders - Drug Targets.2024; 23(6): 746. CrossRef - Diet choices determine mercury exposure risks for people living in gold mining regions of Peru
Melissa J Marchese, Jacqueline R Gerson, Axel J Berky, Charles Driscoll, Luis E Fernandez, Heileen Hsu-Kim, Kelsey N Lansdale, Eliza Letourneau, Mario Montesdeoca, William K Pan, Emily Robie, Claudia Vega, Emily S Bernhardt
Environmental Research: Health.2024; 2(3): 035001. CrossRef - Bioaccumulation of Lead and Mercury in Water, Sediment, and Fish Samples of Baraila Lake, Vaishali, Bihar
Saima Anjum, Anupma Kumari
Biological Trace Element Research.2024;[Epub] CrossRef - Modelling and optimising the performance of graphene oxide-Cu2SnS3-polyaniline nanocomposite as an adsorbent for mercury ion removal
Sara Enferadi, Mohammad Eftekhari, Mohammad Gheibi, Nikoo Nabizadeh Moghaddam, Stanislaw Wacławek, Kourosh Behzadian
Environmental Science and Pollution Research.2024; 31(26): 38196. CrossRef - A naphthalimide-based fluorescent and colorimetric probe for the detection of mercury(II) ions in aqueous solutions and in living cells
Anna S. Polyakova, Pavel A. Panchenko, Anastasija V. Efremenko, Alexey V. Feofanov, Yuri V. Fedorov, Olga A. Fedorova
Mendeleev Communications.2024; 34(3): 418. CrossRef - Impact of Artisanal Gold Mining in Community Conserved Areas with High Biodiversity Using a Multi-Criteria Approach: A Case Study in Colombia
Franco Hernan Gomez, Natalia Pelegri, Juan Guillermo Lopez, Kelly Cristina Torres, Mentore Vaccari
Pollutants.2024; 4(2): 276. CrossRef - How the Western Diet Thwarts the Epigenetic Efforts of Gut Microbes in Ulcerative Colitis and Its Association with Colorectal Cancer
Avisek Majumder, Shabana Bano
Biomolecules.2024; 14(6): 633. CrossRef - An evaluation of fish and invertebrate mercury concentrations in the Caribbean Region
Linroy D. Christian, Mark E. H. Burton, Azad Mohammed, Wendy Nelson, Tahlia Ali Shah, Laël Bertide-Josiah, Helen G. Yurek, David C. Evers
Ecotoxicology.2024; 33(4-5): 397. CrossRef - Determination of Low Concentrations of Mercury Based on the Electrodeposition Time
Kenshin Takemura, Wataru Iwasaki, Nobutomo Morita, Shinya Ohmagari, Yasunori Takaki, Hitomi Fukaura, Kazuya Kikunaga
Nanomaterials.2024; 14(11): 981. CrossRef - Maternal diet quality during pregnancy and biomarkers of potentially toxic trace element exposure: Data from the ELFE cohort
Courtney Dow, Manik Kadawathagedara, Manel Ghozal, Marie-Aline Charles, Karine Adel-Patient, Clémentine Dereumeaux, Blandine de Lauzon-Guillain
Food and Chemical Toxicology.2024; 190: 114793. CrossRef - Animal waste as a valuable biosorbent in the removal of heavy metals from aquatic ecosystem—an eco-friendly approach
Arti Sharma, Isha Devi
Environmental Monitoring and Assessment.2024;[Epub] CrossRef - Traceable Calibration of Atmospheric Oxidized Mercury Measurements
Tyler R. Elgiar, Seth N. Lyman, Teodor D. Andron, Lynne Gratz, A. Gannet Hallar, Milena Horvat, Sreekanth Vijayakumaran Nair, Trevor O’Neil, Rainer Volkamer, Igor Živković
Environmental Science & Technology.2024; 58(24): 10706. CrossRef - Quantifying heavy metal and radionuclide contamination in fish and water proximal to a uranium tailings facility: A Linshui River basin investigation, China
Yan Jin, Bo Fu, Xiaofeng Wang
Journal of Trace Elements in Medicine and Biology.2024; 85: 127485. CrossRef - Watershed features shape spatial patterns of fish tissue mercury in a boreal river network
David W. French, Daniel E. Schindler, Sean R. Brennan, Gordon W. Holtgrieve
Science of The Total Environment.2024; 945: 174060. CrossRef - Potential Non-carcinogenic and Carcinogenic Health Risks of Elements of Health Concern Bioaccumulated in Seafood from Local Fish Rafts in Trang Province, Thailand
Kanjana Imsilp, Niyada Lansubsakul, Wachiryah Thong-asa, Pattanasuda Sirinupong, Pun Yeesin, Napasorn Phaochoosak, Phanwimol Tanhan
Journal of Agriculture and Food Research.2024; : 101272. CrossRef - A Novel Rhodamine Probe Acting as Chemosensor for Selective Recognition of Cu2+ and Hg2+ Ions: An Experimental and First Principle Studies
Pawan Kumar Sada, Amit Bar, Amanpreet Kaur Jassal, Prabhat Kumar, S. Srikrishna, Alok Kumar Singh, Sumit Kumar, Laxman Singh, Abhishek Rai
Journal of Fluorescence.2023;[Epub] CrossRef - Effect of a 110 ppb mercury exposition on neotropical bumble bee workers, Bombus atratus: in situ localization of Hsp70 and Hsp90 and general morphological changes of hepato-nephrocitic cells
Paulo José Balsamo, Felipe Lissoni de Andrade Nogueira, Leticia Ceschi-Bertoli, Raquel Fernanda Salla, Fabiana Martins Costa Maia, Silvia Pierre Irazusta, Guilherme Andrade Neto Schmitz Boeing, Fábio Camargo Abdalla
Journal of Apicultural Research.2023; 62(4): 953. CrossRef - Brain Injury Induced by Mercury in Common Carp: Novel Insight from Transcriptome Analysis
Yue Zhang, Yuting Lu, Peijun Zhang, Xinchi Shang, Yuehong Li
Biological Trace Element Research.2023; 201(1): 403. CrossRef - Assessment of the consumptive safety of mercury in fish from the surface waters of the Vologda region in northwestern Russia
Elena Ivanova, Liubov Eltsova, Victor Komov, Mikhail Borisov, Nikolay Tropin, Samanta Borboshova, Olga Rumiantseva, Victoria Petrova, Yuri Udodenko
Environmental Geochemistry and Health.2023; 45(3): 863. CrossRef - Unraveling the chemistry of ionic liquid mediated carbon dots as sensing probe – A review
Hafiz Muhammad Junaid, Shahid Munir, Madeeha Batool
Trends in Environmental Analytical Chemistry.2023; 40: e00214. CrossRef - Effect of exogenous and endogenous sulfide on the production and the export of methylmercury by sulfate-reducing bacteria
Sophie Barrouilhet, Mathilde Monperrus, Emmanuel Tessier, Bahia Khalfaoui-Hassani, Rémy Guyoneaud, Marie-Pierre Isaure, Marisol Goñi-Urriza
Environmental Science and Pollution Research.2023; 30(2): 3835. CrossRef - Mercury waste from artisanal and small-scale gold mining facilities: a risk to farm ecosystems—a case study of Obuasi, Ghana
Sylvester Addai-Arhin, Randy Novirsa, Huiho Jeong, Quang Dinh Phan, Nana Hirota, Yasuhiro Ishibashi, Hideki Shiratsuchi, Koji Arizono
Environmental Science and Pollution Research.2023; 30(2): 4293. CrossRef - Determination and speciation of methyl mercury and total mercury in fish tissue samples by gold-coated W-coil atom trap cold vapor atomic absorption spectrometry
Muhammet Atasoy, Dilek Yildiz, İbrahim Kula, Ali İmran Vaizoğullar
Food Chemistry.2023; 401: 134152. CrossRef - Vinyl substituted triphenylamine based turn-off fluorescent probe for selective and sensitive detection of mercury (II) in water and live cells
Nishant Kumar Choudhary, Lavanya L. Mittapelli, Pritam Kumar Roy, Gourav Das, Mahitosh Mandal, Kiran R. Gore
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy.2023; 285: 121887. CrossRef - Deep Eutectic Solvent Based Liquid-Liquid Microextraction of Mercury in Water, Hair and Fish with Spectrophotometric Determination: A Green Protocol
Mansoor Khan, Mustafa Soylak
Analytical Letters.2023; 56(7): 1161. CrossRef - Novel Determination of Elemental Mercury in Silicate Rock by Thermal Desorption
L. Ghezzi, M. Valerio, R. Petrini
Analytical Letters.2023; 56(8): 1270. CrossRef - The association between exposure to multiple toxic metals and the risk of endometriosis: Evidence from the results of blood and follicular fluid
Lingchao Shen, Chunmei Liang, Danyang Li, Zhikang Zhang, Xin Wang, Tingting Jiang, Xun Su, Tao Yin, Weiwei Zou, Xiaolei Wang, Yajing Liu, Dan Liang, Zhaolian Wei, Yunxia Cao, Dongmei Ji
Science of The Total Environment.2023; 855: 158882. CrossRef - A high-efficiency decomposition method for mono and dimethylmercury induced by low-energy electron attachment (<≈7 eV): A computational insight into the decomposition mechanism of extremely toxic mercury compounds
Saba Hadidi
Chemosphere.2023; 310: 136845. CrossRef - Neuropsychological effects and cognitive deficits associated with exposure to mercury and arsenic in children and adolescents of the Mojana region, Colombia
César Argumedos De la Ossa, Andrés Fernando Ramírez-Giraldo, Katy Arroyo-Alvis, José Marrugo-Negrete, Sergi Díez
Environmental Research.2023; 216: 114467. CrossRef - Fungal necromass presents a high potential for Mercury immobilization in soil
François Maillard, Stéphane Pflender, Katherine A. Heckman, Michel Chalot, Peter G. Kennedy
Chemosphere.2023; 311: 136994. CrossRef - Timing and magnitude of net methylmercury effects on waterbird reproductive output are dependent on food availability
Jabi Zabala, Joel C. Trexler, Nilmini Jayasena, Peter Frederick
Science of The Total Environment.2023; 858: 159706. CrossRef - Luteolin alleviates inorganic mercury-induced liver injury in quails by resisting oxidative stress and promoting mercury ion excretion
Yan Liu, Xinyu Guo, Lu Yu, Yuxiang Huang, Changming Guo, Siyu Li, Xu Yang, Zhigang Zhang
Molecular Biology Reports.2023; 50(1): 399. CrossRef - Trophic structure and biomagnification of cadmium, mercury and selenium in brown smooth hound shark (Mustelus henlei) within a trophic web
Laura María Pantoja-Echevarría, Ana Judith Marmolejo-Rodríguez, Felipe Galván-Magaña, Fernando R. Elorriaga-Verplancken, Arturo Tripp-Valdéz, Elena Tamburin, Ariagna Lara, M.P. Jonathan, S.B. Sujitha, Antonio Delgado-Huertas, Laura Arreola-Mendoza
Food Webs.2023; 34: e00263. CrossRef - Total mercury, methylmercury, and selenium concentrations in blue marlin Makaira nigricans from a long-term dataset in the western north Atlantic
P.J. Rudershausen, F.A. Cross, B.J. Runde, D.W. Evans, W.G. Cope, J.A. Buckel
Science of The Total Environment.2023; 858: 159947. CrossRef - A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration
N. Nivetha, B. Srivarshine, B. Sowmya, Mangaiyarkarasi Rajendiran, Panchamoorthy Saravanan, R. Rajeshkannan, M. Rajasimman, Thi Hong Trang Pham, VenkatKumar Shanmugam, Elena-Niculina Dragoi
Chemosphere.2023; 312: 137099. CrossRef - Contamination and health risks of trace metals in water and sediments of May Sieley stream, Ethiopia
Elias Habineza, Rodgers Makwinja, Yoshihiko Inagaki
Physics and Chemistry of the Earth, Parts A/B/C.2023; 129: 103315. CrossRef - Ferrous sulfide nanoparticles control mercury speciation and bioavailability to methylating bacteria in contaminated groundwater: Impacts of mercury species
Yanyan Gong, Jie Yin, Tong Zhang, Weizhao Yin, Luyao Sun, Qiru Liang, Qilin Wang
Chemical Engineering Journal.2023; 455: 140612. CrossRef - Suicidal intoxication with mercury chloride
Sławomir Majdanik, Barbara Potocka-Banaś, Sebastian Glowinski, Sylwester Luzny
Forensic Toxicology.2023; 41(2): 304. CrossRef - N-doped carbon nanospheres as selective fluorescent probes for mercury detection in contaminated aqueous media: chemistry, fluorescence probing, cell line patterning, and liver tissue interaction
Soheil Sojdeh, Ali Banitalebi Dehkordi, Alireza Badiei, Ali Zarrabi, Pooyan Makvandi, Milad Ashrafizadeh, Mohammad Reza Saeb, Eder C. Lima, Mohammad Rabiee, Mohsen Asadnia, Thomas J. Webster, Navid Rabiee
Environmental Science and Pollution Research.2023; 30(14): 40327. CrossRef - Synthesis of a Novel Hydrazone Functionality based Spectrophotometric Probe for Selective and Sensitive Estimation of Toxic Heavy Metal Ions
Zainab Amin, Tabasum Rauf, Qounsar Jan, Mohammad Yaseen Kuchey, Feroz Ahmad Sofi, Tabasum Ismail, Auqib Rashid, Bilal Ahmad Bhat, Naheed Sidiq, Mohsin Ahmad Bhat
ChemistrySelect.2023;[Epub] CrossRef - Kanser Gelişiminde Ağır Metallerin Rolü
Nebiye Pelin TÜRKER
International Journal of Life Sciences and Biotechnology.2023; 6(1): 101. CrossRef - A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals
Sana Ullah, Shahid Ahmad, Xinle Guo, Saleem Ullah, Sana Ullah, Ghulam Nabi, Kunyuan Wanghe
Frontiers in Endocrinology.2023;[Epub] CrossRef - Naked-eye colorimetric and switch-on fluorescence chemosensor based on a rhodamine derivative for Hg2+: Smartphone device, test-kit and food sample applications
Hayriye Nevin Genc, Ozlem Guctekin Yasar, Sukriye Nihan Karuk Elmas, Fatma Nur Arslan, Ibrahim Yilmaz, Abdulkadir Sirit
Journal of Photochemistry and Photobiology A: Chemistry.2023; 438: 114558. CrossRef - Critical review on biogeochemical dynamics of mercury (Hg) and its abatement strategies
Arun Dev Singh, Kanika Khanna, Jaspreet Kour, Shalini Dhiman, Tamanna Bhardwaj, Kamini Devi, Neerja Sharma, Pardeep Kumar, Nitika Kapoor, Priyanka Sharma, Priya Arora, Anket Sharma, Renu Bhardwaj
Chemosphere.2023; 319: 137917. CrossRef - Characterization of childhood exposure to environmental contaminants using stool in a semi-urban middle-class cohort from eastern Canada.
Félix Hardy, Larissa Takser, Viginie Gillet, Andrea A. Baccarelli, Jean-Philippe Bellenger
Environmental Research.2023; 222: 115367. CrossRef - A Recent Update on Rhodamine Dye Based Sensor Molecules: A Review
Soma Sarkar, Abhik Chatterjee, Kinkar Biswas
Critical Reviews in Analytical Chemistry.2023; : 1. CrossRef - Lactic acid bacteria strains reduce in vitro mercury toxicity on the intestinal mucosa
Pilar Rodríguez-Viso, Adrián Domene, Dinoraz Vélez, Vicenta Devesa, Manuel Zúñiga, Vicente Monedero
Food and Chemical Toxicology.2023; 173: 113631. CrossRef - Potentially Harmful Element toxicity in Geophagic clays consumed in parts of southeastern Nigeria
Jerry O. Olajide-Kayode, Tesleem O. Kolawole, Opeoluwa O. Oyaniran, Shakirat O. Mustapha, Akinade S. Olatunji
Journal of Trace Elements and Minerals.2023; 4: 100050. CrossRef - Investigation of heavy metal contamination and associated health risks in groundwater sources of southwestern Punjab, India
Pargin Bangotra, Rajan Jakhu, Mukesh Prasad, R. S. Aswal, Ansumali Ashish, Zainab Mushtaq, Rohit Mehra
Environmental Monitoring and Assessment.2023;[Epub] CrossRef - Mercury Exposure from the Consumption of Dietary Supplements Containing Vegetable, Cod Liver, and Shark Liver Oils
Barbara Brodziak-Dopierała, Agnieszka Fischer, Martyna Chrzanowska, Bożena Ahnert
International Journal of Environmental Research and Public Health.2023; 20(3): 2129. CrossRef - Molecular Amplification as an Affordable Strategy for Trace‐Level Detection of Ionic Analytes with Fluorimetric or Colorimetric Readout
Abhijnan Ray Choudhury, Nilanjan Dey
ChemPhotoChem.2023;[Epub] CrossRef - Mercury ions impact the kinetic and thermal stabilities of human lens γ-crystallins via direct metal-protein interactions
Oscar Rodríguez-Meza, Giovanni Palomino-Vizcaino, Liliana Quintanar, Miguel Costas
Journal of Inorganic Biochemistry.2023; 242: 112159. CrossRef - Identification of Mercury Emissions in Soot with the Quadrant Method on Combustion of Gold in Aceh Jaya District
Lensoni Lensoni, M. Adlim, H. Kamil, T. Karma, Suhendrayatna Suhendrayatna
Open Access Macedonian Journal of Medical Sciences.2023; 11(E): 29. CrossRef - Phytoremediation Potential of Heavy Metals by Cyperus rotundus
Sachini P. Ariyachandra, Iustus S. Alwis, Eranga M. Wimalasiri
Reviews in Agricultural Science.2023; 11: 20. CrossRef - Chronic Mercury Exposure and GSTP1 Polymorphism in Munduruku Indigenous from Brazilian Amazon
Mayara Calixto da Silva, Rogério Adas Ayres de Oliveira, Ana Claudia Santiago de Vasconcellos, Bruno Hojo Rebouças, Bruna Duarte Pinto, Marcelo de Oliveira Lima, Iracina Maura de Jesus, Daniel Escorsim Machado, Sandra Souza Hacon, Paulo Cesar Basta, Jamil
Toxics.2023; 11(2): 138. CrossRef - Our evolved understanding of the human health risks of mercury
Niladri Basu, Ashley Bastiansz, José G. Dórea, Masatake Fujimura, Milena Horvat, Emelyn Shroff, Pál Weihe, Irina Zastenskaya
Ambio.2023; 52(5): 877. CrossRef - Neutron Activation Analysis: An Excellent Nondestructive Analytical Technique for Trace Metal Analysis
Debashree Debasish Das, Nikita Sharma, Pooja A Chawla
Critical Reviews in Analytical Chemistry.2023; : 1. CrossRef - Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats
Xi Wu, Ping Li, Junyan Tao, Xiong Chen, Aihua Zhang
Toxics.2023; 11(2): 191. CrossRef - Mercury Levels in Sediment, Water and Selected Organisms Collected in a Coastal Contaminated Environment: The Marano and Grado Lagoon (Northern Adriatic Sea, Italy)
Nicola Bettoso, Federico Pittaluga, Sergio Predonzani, Antonella Zanello, Alessandro Acquavita
Applied Sciences.2023; 13(5): 3064. CrossRef - Heavy Metals Influence on the Bacterial Community of Soils: A Review
Ivan Sazykin, Ludmila Khmelevtsova, Tatiana Azhogina, Marina Sazykina
Agriculture.2023; 13(3): 653. CrossRef - Apitoxin alleviates methyl mercury-induced peripheral neurotoxicity in male rats by regulating dorsal root ganglia neuronal degeneration and oxidative stress
Moustafa S. Abdelhamid, Khlood M. El.Bohi, Mohamed H. Sherif, Manar S. Abdelhamid, Mohamed M. Abdel-Daim, Yaser H.A. Elewa, Mohamed M.M. Metwally, Ghadeer M. Albadrani, Agnieszka Najda, Shereen El. Abdel-Hamid, Ehsan H. Abu-Zeid
Biomedicine & Pharmacotherapy.2023; 161: 114521. CrossRef - Detection of Lysosomal Hg2+ Using a pH-Independent Naphthalene Monoimide-Based Fluoroprobe
Rupam Roy, Tanoy Dutta, Shruti Nema, Apurba Lal Koner
Chemosensors.2023; 11(3): 184. CrossRef - Nanoplastics pose a greater effect than microplastics in enhancing mercury toxicity to marine copepods
Zhuoan Bai, Yu Zhang, Luman Cheng, Xiaoping Zhou, Minghua Wang
Chemosphere.2023; 325: 138371. CrossRef - Regional geochemistry of mercury in the Sino-Mongolian border region
Wei Wang, Lanshi Nie, Xinbin Cheng, Hanliang Liu, Shojin Davaa, Xueqiu Wang, Qinghua Chi, Jian Zhou, Qinghai Hu, Xuemiao Du
Applied Geochemistry.2023; 151: 105628. CrossRef - Geochemical assessment and pollution evaluation of stream sediments’ quality impacted by industrial activities at Suame Magazine area, Kumasi, Ghana
Josephine Adu-Gyamfi, Emmanuel Kwesi Nyantakyi, Julius Kwame Borkloe, Prodeo Yao Agbotui, Saeed Ibn Idris Kofi Yeboah, Nana Osei Bonsu Ackerson, Emmanuel Acheaw, Clement Apuri Wezenamo, Martin Kyereh Domfeh, Emmanuel Nsiah, Thomas Ntori, Ebenezer Gyamfi
Arabian Journal of Geosciences.2023;[Epub] CrossRef - Mercury Accumulation in Food Crops and Phytoremediation Potential of Wild Plants Thriving in Artisanal and Small-Scale Gold Mining Areas in Uganda
Jamilu E. Ssenku, Betty Naziriwo, Jennifer Kutesakwe, Abubakar Sadik Mustafa, Derrick Kayeera, Emmanuel Tebandeke
Pollutants.2023; 3(2): 181. CrossRef - Combined effects of climate change and environmentally relevant mixtures of endocrine disrupting compounds on the fitness and gonads' maturation dynamics of Nucella lapillus (Gastropoda)
H. Morais, F. Arenas, C. Cruzeiro, S. Galante-Oliveira, P.G. Cardoso
Marine Pollution Bulletin.2023; 190: 114841. CrossRef - Design of Fluorescent Carbon Dots (CDs) for the Selective detection of Metal‐Containing Ions
Alejandro López‐Beltrán, Claudia Iriarte‐Mesa, Clarissa Murru, Frank J. Chao‐Mujica, Angel Luis Corcho‐Valdés, Lisandra Morales‐Álvarez, Luis Felipe Desdín‐García, Johnny Deschamps, Manuel Antuch
Chemistry – A European Journal.2023;[Epub] CrossRef - Disease-associated metabolic pathways affected by heavy metals and metalloid
Zinia Haidar, Kaniz Fatema, Sabrina Samad Shoily, Abu Ashfaqur Sajib
Toxicology Reports.2023; 10: 554. CrossRef - Dual responsive pyridoxal-AHMT based fluorescent sensor towards zinc(ii) and mercury(ii) ions and its bioimaging application
Kettalu Ananthan Karthick, Bhaskaran Shankar, Santhalingam Gayathri, Manikka Kubendran Aravind, Balasubramaniem Ashokkumar, Arunachalam Tamilselvi
New Journal of Chemistry.2023; 47(19): 9427. CrossRef - Impact of clay mineralogy on the petrophysical properties of tight sandstones
Hamad S. Al-Kharra'a, Karl-Heinz A.A. Wolf, Abdulrahman A. AlQuraishi, Mohamed A. Mahmoud, Ivan Deshenenkov, Mohammed A. AlDuhailan, Sulaiman A. Alarifi, Naif B. AlQahtani, Hyung T. Kwak, Pacelli L.J. Zitha
Geoenergy Science and Engineering.2023; 227: 211883. CrossRef - Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review
Aniruddha Sarker, Md Abdullah Al Masud, Deen Mohammad Deepo, Kallol Das, Rakhi Nandi, Most Waheda Rahman Ansary, Abu Reza Md Towfiqul Islam, Tofazzal Islam
Chemosphere.2023; 332: 138861. CrossRef - Seasonal variation of mercury in settled dust from brick kiln pollution in Sonora, Mexico: Ecological risk and human health implication
Benedetto Schiavo, Diana Meza-Figueroa, Ofelia Morton-Bermea, Efrain Vizuete-Jaramillo, Agustin Robles-Morua
Atmospheric Pollution Research.2023; 14(7): 101787. CrossRef - Drinking water quality and inflammatory bowel disease: a prospective cohort study
Shuduo Zhou, Pengfei Chai, Xuejie Dong, Zhisheng Liang, Zongming Yang, Junxia Li, Guigen Teng, Shengzhi Sun, Ming Xu, Zhi-Jie Zheng, Jianbing Wang, Zhenyu Zhang, Kun Chen
Environmental Science and Pollution Research.2023; 30(27): 71171. CrossRef - Intelligent detection strategy and bioimaging application of dual-responsive Hg2+ and ONOO− using near-infrared probes
Xiao Wang, Xuechuan Wang, Qingxin Han
Analytica Chimica Acta.2023; 1266: 341358. CrossRef - Nonfatal Occupational Injuries Among Artisanal and Small-scale Gold Mining Workers in Ethiopia
Fentayehu Abebil, Yifokire Tefera, Worku Tefera, Abera Kumie, Hailemichael Mulugeta, Genanew Kassie
Environmental Health Insights.2023; 17: 117863022311718. CrossRef - Interaction of mercury species with proteins: towards possible mechanism of mercurial toxicology
Sharmin Akther Rupa, Md Abdul Majed Patwary, Mohammed Mahbubul Matin, William Emmanuel Ghann, Jamal Uddin, Mohsin Kazi
Toxicology Research.2023; 12(3): 355. CrossRef - Potential ecological risk assessment of heavy metals associated with abattoir liquid waste: A narrative and systematic review
Solomon Nandomah, Isaac Kow Tetteh
Heliyon.2023; 9(8): e17359. CrossRef - Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia)
Federico Floreani, Elena Pavoni, Mateja Gosar, Stefano Covelli
Atmosphere.2023; 14(6): 1036. CrossRef - Mercury impact on wildlife: An analysis of the literature
Mukta Singh, Rahul Kanaoujiya, Meenakshi, Shekhar Srivastava
Materials Today: Proceedings.2023;[Epub] CrossRef - The potential of microorganisms as biomonitoring and bioremediation tools for mercury-contaminated soils
Lorraine Meyer, Stéphane Guyot, Michel Chalot, Nicolas Capelli
Ecotoxicology and Environmental Safety.2023; 262: 115185. CrossRef - Nitrogen-doped fluorescent active fullerenes as a fluorescent probe for the detection of Hg2+ ions in aqueous solutions
Sahil, Suresh Kumar, Yash B. Barot, Roli Mishra, Dilbag Singh, Neeraj Gupta
Environmental Nanotechnology, Monitoring & Management.2023; 20: 100845. CrossRef - Metal free synthesis of 2,3-dideoxy-α, β-unsaturated carbohydrate enals (Perlin aldehydes)
Kavita Singh, Sourav Sagar Behera, Rajdeep Tyagi, Ghanshyam Tiwari, Ram Sagar
Carbohydrate Research.2023; 531: 108890. CrossRef - Thiophene functionalized cellulose immobilized with metal organic framework for removal of heavy metals
Alaa M. Munshi, Nasser A. Alamrani, Hussain Alessa, Meshari Aljohani, Saham F. Ibarhiam, Fawaz A. Saad, Salhah D. Al-Qahtani, Nashwa M. El-Metwaly
Cellulose.2023; 30(11): 7235. CrossRef - Mercury contamination in seafood from an aquatic environment impacted by anthropic activity: seasonality and human health risk
Paloma de Almeida Rodrigues, Júlia Vianna de Pinho, Alexandre Mendes Ramos-Filho, Gustavo Lata Neves, Carlos Adam Conte-Junior
Environmental Science and Pollution Research.2023; 30(36): 85390. CrossRef - Layer by layer assembly CS/PAA for high sensitivity detection of heavy metal ions using mode interference sensor
Minglu Yan, Ruiduo Wang, Beibei Liu, Yangyang Li, Yarong Li, Man Jiang
Optics Communications.2023; 545: 129725. CrossRef - Mercury Intake Estimation in Adult Individuals from Trieste, Italy: Hair Mercury Assessment and Validation of a Newly Developed Food Frequency Questionnaire
Andrea De Giovanni, Vincenzo Iannuzzi, Gianni Gallello, Cristina Giuliani, Mauro Marini, M. Luisa Cervera, Donata Luiselli
Pollutants.2023; 3(3): 320. CrossRef - Nuclear waste as a socio-technical problem
Sven Ove Hansson
TATuP - Zeitschrift für Technikfolgenabschätzung in Theorie und Praxis.2023; 32(2): 50. CrossRef - Stimuli-Responsive Copolymer-Mediated Synthesis of Gold Nanoparticles for Nanozyme-Based Colorimetric Detection of Mercury(II) Ions
Namitha K. Preman, Supriya Jain, Anila Antony, Dhanya M. Shetty, Nasrin Fathima, K. Sudhakara Prasad, Renjith P. Johnson
ACS Applied Polymer Materials.2023; 5(8): 6377. CrossRef - Relationship between trace element concentrations and body length in dolphinfish (Coryphaena hippurus) in the northwest Atlantic Ocean
Jessica Dutton
Environmental Science and Pollution Research.2023; 30(37): 87757. CrossRef - Mercury bioaccessiblity in freshwater fish species from northern Canada
Sara Packull-McCormick, Alicia Cowan, Ken D. Stark, Mike Low, Mary Gamberg, Heidi Swanson, Brian Laird
Science of The Total Environment.2023; 899: 165624. CrossRef - Assessment of heavy metal contamination in street dust: concentrations, bioaccessibility, and human health risks in coal mine and thermal power plant complex
Mala Kumari, Abhishek Kumar, Tanushree Bhattacharya
Environmental Geochemistry and Health.2023; 45(10): 7339. CrossRef - Silver nanoparticles deposited carbon microspheres nanozyme with enhanced peroxidase-like catalysis for colorimetric detection of Hg2+ in seafood
Ziyi Zhang, Dan Liu, Xiaoshuo Zhang, Xueli Luo, Wanmei Lin, Zhonghong Li, Jihong Huang
Microchimica Acta.2023;[Epub] CrossRef - A Simple Live Cell Imaging “Turn–On” Fluorescence Probe for the Selective and Sensitive Detection of Aqueous Hg2+ Ions
Ajayakumar Aswathy, Pookalavan Karicherry Vineetha, Vishal Kandathil, Jiya Jose, Sarita G. Bhat, Narayanapillai Manoj
Journal of Fluorescence.2023;[Epub] CrossRef - Maternal provisioning interacts with incubation temperature to affect hatchling mercury exposure in an oviparous reptile
Josiah M. Johnson, Christopher R. Smaga, Samantha L. Bock, Benjamin B. Parrott
Biology Letters.2023;[Epub] CrossRef - Mercury Concentrations in Whale Shark (Rhincodon typus) Embryo Muscle Tissue
Jessica Dutton, Jessica C. Hobbs, Shoou-Jeng Joung, Jennifer V. Schmidt
Bulletin of Environmental Contamination and Toxicology.2023;[Epub] CrossRef - Variation in habitat use and its consequences for mercury exposure in two Eastern Ontario bat species, Myotis lucifugus and Eptesicus fuscus
Bailey Bedard, Brian Hickey, John Chételat, Jan A. Mennigen
Ecotoxicology.2023; 32(7): 845. CrossRef - Tailored ZnO nanostructures for efficient sensing of toxic metallic ions of drainage systems
Km. Preeti, Anirudh Kumar, Naini Jain, Ajeet Kaushik, Yogendra Kumar Mishra, Sanjeev K. Sharma
Materials Today Sustainability.2023; 24: 100515. CrossRef - A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects
Vinay Kumar, Mridul Umesh, Manoj Kumar Shanmugam, Pritha Chakraborty, Lucky Duhan, Sathyanarayana N. Gummadi, Ritu Pasrija, Iyyappan Jayaraj, Lohith Kumar Dasarahally Huligowda
Sustainability.2023; 15(18): 13292. CrossRef - High levels of mercury contamination in a necrophage dung beetle of the Amazon rainforest (Scarabaeidae: Scarabaeinae)
Elena Chaboteaux, Alejandro Lopera Toro, Claudia Maribel Vega, Adrian Forsyth
Biotropica.2023; 55(6): 1081. CrossRef - Keystone seabird may face thermoregulatory challenges in a warming Arctic
Melissa L. Grunst, Andrea S. Grunst, David Grémillet, Akiko Kato, Sophie Gentès, Jérôme Fort
Scientific Reports.2023;[Epub] CrossRef - Unveiling the molecular mechanisms and developmental consequences of mercury (Hg) toxicity in zebrafish embryo-larvae: A comprehensive approach
Magda Carvalho Henriques, Inês Carvalho, Cátia Santos, Maria Teresa Herdeiro, Margarida Fardilha, Maria Dimitriou Pavlaki, Susana Loureiro
Neurotoxicology and Teratology.2023; 100: 107302. CrossRef - Fluorescent dendritic fibrous nanosilica/polyurethane composite membranes for selective and sensitive recognition and removal of Hg2+/Hg+ ions
Jiao He, Siyuan Zhang, Xinjian Cheng
Chemical Engineering Journal.2023; 476: 146707. CrossRef - Biochemical and structural basis of mercuric reductase, GbsMerA, from Gelidibacter salicanalis PAMC21136
Bashu Dev Pardhe, Min Ju Lee, Jun Hyuck Lee, Hackwon Do, Tae-Jin Oh
Scientific Reports.2023;[Epub] CrossRef - A mitochondrion-targeted fluorescent probe based on ESIPT phthalimide for the detection of Hg2+ with large Stokes shift
Aishan Ren, Wenqin Yao, Dongjian Zhu
The Analyst.2023; 148(23): 5882. CrossRef - Occurrence, toxicity load, pollution index and health risk assessment of trace elements in drinking water of two catchments in North-western Himalayas
Asiya Rahim, Savidh Khan, Asha Rani, Vysetti Balaram, Rayees Ahmed
International Journal of River Basin Management.2023; : 1. CrossRef - Toxic metals in pregnancy and congenital heart defects. Insights and new perspectives for a technology-driven reduction in food sources
Francesca Gorini, Alessandro Tonacci
Exploration of Cardiology.2023; 1(3): 114. CrossRef - Exploring the origins and cleanup of mercury contamination: a comprehensive review
Davamani Veeraswamy, Arulmani Subramanian, Deepasri Mohan, Parameswari Ettiyagounder, Paul Sebastian Selvaraj, Sangeetha Piriya Ramasamy, Venkatesan Veeramani
Environmental Science and Pollution Research.2023;[Epub] CrossRef - Education and equipment distribution lead to increased mercury knowledge and retort use in artisanal and small-scale gold mining communities in Senegal
Arabella Chen, Falaye Danfakha, Heidi Hausermann, Jacqueline R. Gerson
Cleaner Production Letters.2023; 5: 100050. CrossRef - A Reaction-Based ESIPT Fluorescent Probe for the Detection of Hg2+ with Large Stokes Shift
Dongjian Zhu, Wenqin Yao, Aishan Ren
Journal of Fluorescence.2023;[Epub] CrossRef - Mercury Ion-Responsive Coalescence of Silver Nanoparticles on a Silica Nanoparticle Core for Surface-Enhanced Raman Scattering Sensing
Yoon-Hee Kim, Eunil Hahm, Xuan-Hung Pham, Homan Kang, Dae-Hong Jeong, Hyejin Chang, Bong-Hyun Jun
ACS Applied Nano Materials.2023; 6(24): 23469. CrossRef - Heterogeneous Reaction of Gaseous Mercuric Chloride with Atmospherically Relevant Organic Films
Alexei F. Khalizov, Na Mao
ACS Earth and Space Chemistry.2023; 7(12): 2593. CrossRef - Mercury and Autism Spectrum Disorder: Exploring the Link through Comprehensive Review and Meta-Analysis
Aleksandar Stojsavljević, Novak Lakićević, Slađan Pavlović
Biomedicines.2023; 11(12): 3344. CrossRef - Physical Characterization and Structure Elucidation of Exopolysaccharide from Marine Halotolerant Bacterium Enterobacter cloacae VHP-34
Vishal H Patel, Kamlesh Shah, Falguni R Patel
Industrial Biotechnology.2023; 19(6): 326. CrossRef - Exposure to mercury in dentistry: A safety concern
Pushparaja Shetty, Akshatha Shetty
Acta stomatologica Naissi.2023; 39(87): 2639. CrossRef - EFECTOS ECOTÓXICOS DE METALES PESADOS SOBRE Daphnia magna Y Paracheirodon innesi EN UN RÍO DE LA AMAZONÍA PERUANA
Esther Méniz-Oshiro, José Alberto Iannacone Olíver
Acta Biológica Colombiana.2023; 28(3): 492. CrossRef - Metal Ion Detection by Carbon Dots—A Review
Madeeha Batool, Hafiz Muhammad Junaid, Sobia Tabassum, Farah Kanwal, Kamran Abid, Zara Fatima, Asma Tufail Shah
Critical Reviews in Analytical Chemistry.2022; 52(4): 756. CrossRef - Sampling in environmental matrices: a critical review
Prashant P. Bhave, Karan Sadhwani
Environmental Forensics.2022; 23(1-2): 75. CrossRef - Mercury transportation dynamics in the Ganga Alluvial Plain, India: rainwater–groundwater–river water interaction study from hotspot region
V. Devi, M. M. Atique, A. Raju, G. Upreti, D. K. Jigyasu, J. K. Yadav, S. Singh, R. Kar, M. Singh
International Journal of Environmental Science and Technology.2022; 19(6): 4891. CrossRef - The human health risks assessment of mercury in soils and plantains from farms in selected artisanal and small‐scale gold mining communities around Obuasi, Ghana
Sylvester Addai‐Arhin, Randy Novirsa, Hui Ho Jeong, Quang Dinh Phan, Nana Hirota, Yasuhiro Ishibashi, Hideki Shiratsuchi, Koji Arizono
Journal of Applied Toxicology.2022; 42(2): 258. CrossRef - Synthesis and applications of Perovskite in heavy metal ions removal-A brief perspective
G. Jayanthi, Sowrirajan Sumathi, V. Andal
Materials Today: Proceedings.2022; 55: 201. CrossRef - Exploiting the high conjugation capacity of creatinine on 3,3′-dithiodipropionic acid di(N-hydroxysuccinimide ester) functionalized gold nanoparticles towards sensitive determination of mercury (II) ion in water
Titilope John Jayeoye, Tawatchai Kangkamano, Thitima Rujiralai
Journal of Nanostructure in Chemistry.2022; 12(2): 263. CrossRef - Effect of heavy metals: An overview
Kiran, Ruchi Bharti, Renu Sharma
Materials Today: Proceedings.2022; 51: 880. CrossRef - Electronic wastes: A near inexhaustible and an unimaginably wealthy resource for water splitting electrocatalysts
Pitchiah Esakki Karthik, Hashikaa Rajan, Vasanth Rajendiran Jothi, Byoung-In Sang, Sung Chul Yi
Journal of Hazardous Materials.2022; 421: 126687. CrossRef - Mercury and Movement Disorders: The Toxic Legacy Continues
Jacky Ganguly, Dinkar Kulshreshtha, Mandar Jog
Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques.2022; 49(4): 493. CrossRef - Profiling of Circulatory Elements Reveals Alteration of Essential and Toxic Trace Metals in Crohn’s Disease
Aleksandar Stojsavljević, Aleksandra Sokić-Milutinović, Branislav Rovčanin, Ljubiša Tončev, Dragan Manojlović
Biological Trace Element Research.2022; 200(6): 2572. CrossRef - A review on zeolites as cost-effective adsorbents for removal of heavy metals from aqueous environment
E. I. Ugwu, A. Othmani, C. C. Nnaji
International Journal of Environmental Science and Technology.2022; 19(8): 8061. CrossRef - Cellular and genetic mechanism of bacterial mercury resistance and their role in biogeochemistry and bioremediation
Monika Priyadarshanee, Shreosi Chatterjee, Sonalin Rath, Hirak R. Dash, Surajit Das
Journal of Hazardous Materials.2022; 423: 126985. CrossRef - Development of microwave metamaterial-inspired sensors with multiple-band reactivity for mercury contamination detection
Khan Md. Zobayer Hassan, Nantakan Wongkasem
Journal of Electromagnetic Waves and Applications.2022; 36(4): 520. CrossRef - Quantitative Hg2+ detection via forming three coordination complexes using a lysosome targeting quinoline - Fisher aldehyde fluorophore
Selvaraj Muthusamy, Long Zhao, Kanagaraj Rajalakshmi, Dongwei Zhu, Shengjun Wang, John Mack, Kang-Bong Lee, Long Zhang, Weihua Zhu
Talanta.2022; 236: 122884. CrossRef - Assessment of concentration and distribution of total mercury and polychlorinated biphenyls in Green Bay, Wisconsin, USA
Marcia R. Silva, Alice Lecus, Chad Haehle, David Garman, Shelby Brunner
Environmental Science and Pollution Research.2022; 29(9): 13323. CrossRef - Biochemical, physiological (haematological, oxygen-consumption rate) and behavioural effects of mercury exposures on the freshwater snail, Bellamya bengalensis
Kishore Dhara, Shubhajit Saha, Prasenjit Pal, Azubuike V. Chukwuka, Asish Kumar Panigrahi, Nimai Chandra Saha, Caterina Faggio
Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology.2022; 251: 109195. CrossRef - Ultrasensitive determination of mercury by ICP-OES coupled with a vapor generation approach based on solution cathode glow discharge
Zhaoqing Cai, Huijun Zou, Yirui Chen, Zheng Wang
Chinese Chemical Letters.2022; 33(5): 2692. CrossRef - 3,3'-Diselenodipropionic acid (DSePA) forms 1:1 complex with Hg (II) and prevents oxidative stress in cultured cells and mice model
Y. Deshmukh, V.V. Gandhi, B.G. Singh, L.B. Kumbhare, A.K. Debnath, A. Kunwar
Journal of Inorganic Biochemistry.2022; 226: 111638. CrossRef - Trophic position, altitudinal distribution, and water dependence as determining factors for mercury concentrations in tropical montane anurans
Leandro de Oliveira Drummond, Rodrigo Ornellas Meire, Caryne Braga, Carlos Eduardo de Rezende, Olaf Malm, Rui Cerqueira
Science of The Total Environment.2022; 806: 151356. CrossRef - Deciphering the bacterial microbiome in response to long-term mercury contaminated soil
Dongbo Li, Xingjie Li, Yu Tao, Zhenning Yan, Yansong Ao
Ecotoxicology and Environmental Safety.2022; 229: 113062. CrossRef - The myth of vaccination and autism spectrum
Lidia V. Gabis, Odelia Leon Attia, Mia Goldman, Noy Barak, Paula Tefera, Shahar Shefer, Meirav Shaham, Tally Lerman-Sagie
European Journal of Paediatric Neurology.2022; 36: 151. CrossRef - Case studies on longitudinal mercury content in humpback whale (Megaptera novaeangliae) baleen
Carley L. Lowe, Renee Jordan-Ward, Kathleen E. Hunt, Matthew C. Rogers, Alexander J. Werth, Chris Gabriele, Janet Neilson, Frank A. von Hippel, C. Loren Buck
Heliyon.2022; 8(1): e08681. CrossRef - A novel xanthene-based fluorescence turn-on probe for highly selective detection of Hg2+ in water samples and living cells
Zhigang Gao, Siyan Qiu, Minchuan Yan, Haibo Liu, Shaohui Lu, Huihui Lian, Peng Zhang, Jing Zhu, Mingjie Jin
Journal of Molecular Structure.2022; 1254: 132312. CrossRef - New Optimization Understanding of the Removal of Harmful Elements from Gold Tailings: A Review
J. Christophe Niyonzima, Liqun Luo, Ekata Emmanuel Edo, Yanling Xu, Brian Nzuki, Xiaoxue Zhang
JOM.2022; 74(4): 1641. CrossRef - Determination of Mercury, Methylmercury and Selenium Concentrations in Elasmobranch Meat: Fish Consumption Safety
Arianna Storelli, Grazia Barone, Rita Garofalo, Antonio Busco, Maria Maddalena Storelli
International Journal of Environmental Research and Public Health.2022; 19(2): 788. CrossRef - Effects of inorganic mercury exposure in the alveolar bone of rats: an approach of qualitative and morphological aspects
Paula Beatriz de Oliveira Nunes, Maria Karolina Martins Ferreira, Deborah Ribeiro Frazão, Leonardo Oliveira Bittencourt, Victória dos Santos Chemelo, Márcia Cristina Freitas Silva, Armando Lopes Pereira-Neto, Alan Rodrigo Leal Albuquerque, Simone Patricia
PeerJ.2022; 10: e12573. CrossRef - Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity
Saikat Mitra, Arka Jyoti Chakraborty, Abu Montakim Tareq, Talha Bin Emran, Firzan Nainu, Ameer Khusro, Abubakr M. Idris, Mayeen Uddin Khandaker, Hamid Osman, Fahad A. Alhumaydhi, Jesus Simal-Gandara
Journal of King Saud University - Science.2022; 34(3): 101865. CrossRef - Elemental mercury (Hg0) emission, hazards, and control: A brief review
Haipeng Teng, Adnan Raza Altaf
Journal of Hazardous Materials Advances.2022; 5: 100049. CrossRef - SNS donors as mimic to enzymes, chemosensors, and imaging agents
Poonam Kaswan, Preeti Oswal, Arun Kumar, Chandra Mohan Srivastava, Dipti Vaya, Varun Rawat, Kamal Nayan Sharma, Gyandshwar Kumar Rao
Inorganic Chemistry Communications.2022; 136: 109140. CrossRef - Estimation of Atmospheric Dry and Wet Deposition of Particulate Elements at Four Monitoring Sites in the Canadian Athabasca Oil Sands Region
Abdulla Al Mamun, Irene Cheng, Leiming Zhang, Valbona Celo, Ewa Dabek‐Zlotorzynska, Jean‐Pierre Charland
Journal of Geophysical Research: Atmospheres.2022;[Epub] CrossRef - Soil Contamination and Bioaccumulation of Heavy Metals by a Tropical Earthworm Species (Alma nilotica) at Informal E‐Waste Recycling Sites in Douala, Cameroon
Brian Nfor, Patricia Bi Asanga Fai, Simon Awafor Tamungang, Julius N. Fobil, Niladri Basu
Environmental Toxicology and Chemistry.2022; 41(2): 356. CrossRef - Multiple heteroatom-doped photoluminescent carbon dots for ratiometric detection of Hg2+ ions in cell imaging and environmental applications
Sonaimuthu Mohandoss, Hari Datta Khanal, Subramanian Palanisamy, SangGuan You, Jae-Jin Shim, Yong Rok Lee
Analytical Methods.2022; 14(6): 635. CrossRef - Selenium-enriched Bacillus subtilis reduces the effects of mercury-induced on inflammation and intestinal microbes in carp (Cyprinus carpio var. specularis)
Xinchi Shang, Bo Wang, Qingsong Sun, Yue Zhang, Yuting Lu, Shaojun Liu, Yuehong Li
Fish Physiology and Biochemistry.2022; 48(1): 215. CrossRef - Aquatic food animals in the United States: Status quo and challenges
Xingyi Jiang, Yaqi Zhao, Chunya Tang, Megan Appelbaum, Qinchun Rao
Comprehensive Reviews in Food Science and Food Safety.2022; 21(2): 1336. CrossRef - A novel fluorometric chemosensor based on imidazo[4,5-b]phenazine-2-thione for ultrasensitive detection and separation of Hg2+ in aqueous solution
Qing Huang, Qiao Li, Hai-Li Zhang, Wei Zhu, Wen-Juan Qu, Qi Lin, Hong Yao, You-Ming Zhang, Tai-Bao Wei
Canadian Journal of Chemistry.2022; 100(4): 280. CrossRef - The protective effect of nano calcium produced from freshwater clam shells on the histopathological overview of the liver and kidneys of mice exposed to mercury toxins
Sata Yoshida Srie Rahayu, Tri Aminingsih, Ahmad Fudholi
Journal of Trace Elements in Medicine and Biology.2022; 71: 126963. CrossRef - Sensing of mercury ion using light induced aqueous leaf extract mediated green synthesized silver nanoparticles of Cestrum nocturnum L
Pradeep Kumar, Piyush Kumar Sonkar, Kavindra Nath Tiwari, Amit Kumar Singh, Sunil Kumar Mishra, Jyoti Dixit, Vellaichamy Ganesan, Jasmeet Singh
Environmental Science and Pollution Research.2022; 29(53): 79995. CrossRef - Amperometric Detection of Mercury Ions Using Piperazine‐Functionalized Reduced Graphene Oxide as an Efficient Sensing Platform
G. Balu Mahendran, S. Jothi Ramalingam, John Bosco Balaguru Rayappan, Manju Bhargavi Gumpu, Rajendran Ganesh Kumar, Muthaiyan Lakshmanakumar, Noel Nesakumar
ChemistrySelect.2022;[Epub] CrossRef - Contamination, exposure, and health risk assessment of Hg in Pakistan: A review
Sajid Rashid, Izaz Ali Shah, Roberto Xavier Supe Tulcan, Wajid Rashid, Mika Sillanpaa
Environmental Pollution.2022; 301: 118995. CrossRef - Risk assessment for seafood consumers exposed to mercury and other trace elements in fish from Long Island, New York, USA
Xiayan Ye, Cheng-Shiuan Lee, Oliver N. Shipley, Michael G. Frisk, Nicholas S. Fisher
Marine Pollution Bulletin.2022; 176: 113442. CrossRef - High concentrations of HgS, MeHg and toxic gas emissions in thermally affected waste dumps from hard coal mining in Poland
Ádám Nádudvari, Jerzy Cabała, Leszek Marynowski, Mariola Jabłońska, Maria Dziurowicz, Dariusz Malczewski, Barbara Kozielska, Piotr Siupka, Zofia Piotrowska-Seget, Bernd R.T. Simoneit, Mirosław Szczyrba
Journal of Hazardous Materials.2022; 431: 128542. CrossRef - Resveratrol attenuates methylmercury-induced neurotoxicity by modulating synaptic homeostasis
Wenjuan Wang, Caiyun Deng, Fang Chen, Li Zhang, Yi Hu, Qin Lu, Aihua Zhang
Toxicology and Applied Pharmacology.2022; 440: 115952. CrossRef - Comprehensive Review Regarding Mercury Poisoning and Its Complex Involvement in Alzheimer’s Disease
Emanuela Paduraru, Diana Iacob, Viorica Rarinca, Angelica Rusu, Roxana Jijie, Ovidiu-Dumitru Ilie, Alin Ciobica, Mircea Nicoara, Bogdan Doroftei
International Journal of Molecular Sciences.2022; 23(4): 1992. CrossRef - Mercuric chloride‐induced oxidative stress, genotoxicity, haematological changes and histopathological alterations in fish Channa punctatus (Bloch, 1793)
Sunil P. Trivedi, Shefalee Singh, Abha Trivedi, Manoj Kumar
Journal of Fish Biology.2022; 100(4): 868. CrossRef - Salting Reduces Mercury Concentrations in Odontocete Muscle Tissue
Russell Fielding, Kelsie Schiavone, Jessica Dutton
Caribbean Journal of Science.2022;[Epub] CrossRef - Graphitic sulfur functionalized carbon sheets as an efficient “turn-off” absorption probe for the optical sensing of mercury ions in aqueous solutions
Vishal Bharati Jaryal, Dilbag Singh, Neeraj Gupta
New Journal of Chemistry.2022; 46(12): 5712. CrossRef - Mercury Contamination: A Growing Threat to Riverine and Urban Communities in the Brazilian Amazon
Heloisa do Nascimento de Moura Meneses, Marcelo Oliveira-da-Costa, Paulo Cesar Basta, Cristiano Gonçalves Morais, Romulo Jorge Batista Pereira, Suelen Maria Santos de Souza, Sandra de Souza Hacon
International Journal of Environmental Research and Public Health.2022; 19(5): 2816. CrossRef - Quantitative Analysis of the Interactions of Metal Complexes and Amphiphilic Systems: Calorimetric, Spectroscopic and Theoretical Aspects
Rossella Migliore, Tarita Biver, Giampaolo Barone, Carmelo Sgarlata
Biomolecules.2022; 12(3): 408. CrossRef - Dual Fluorometric Detection of Fe3+ and Hg2+ Ions in an Aqueous Medium Using Carbon Quantum Dots as a “Turn-off” Fluorescence Sensor
Shafali Singh, Sushil Kumar Kansal
Journal of Fluorescence.2022; 32(3): 1143. CrossRef - Protecting Black and African Americans from Disproportionate Coal Ash Exposure in Georgia
Julia de Amorim, L.M. Bradley, Nicholas Harbin, Evelyn Kimbrough
Journal of Science Policy & Governance.2022;[Epub] CrossRef - How UNFCCC's COP Can Achieve Carbon Neutrality
Natasha Dacic, Alexa White, Ranveer Ajimal, Katelyn Boisvert, Lunia Oriol, Sivah Akash
Journal of Science Policy & Governance.2022;[Epub] CrossRef - Calculation of mercury intake from the consumption of wild fish by the population of the Vologda region
Elena Sergeevna Ivanova, Lyubov Sergeevna Eltsova, Olesya Petrovna Shuvalova , Viktor Trofimovich Komov , Mikhail Yanovich Borisov
Sanitarnyj vrač (Sanitary Doctor).2022; (3): 226. CrossRef - A systematic review of the concentration of potentially toxic elements in fish from the Persian Gulf: A health risk assessment study
Mehdi Raissy, Mahsa Ansari, Reza Sharafati Chaleshtori, Vahideh Mahdavi, Zahra Hadian, José Manuel Lorenzo, Gea Oliveri Conti, Elcin Huseyn, Amin Mousavi Khaneghah
Food and Chemical Toxicology.2022; 163: 112968. CrossRef - Role of environmental toxicants in the development of hypertensive and cardiovascular diseases
Ehsan Habeeb, Saad Aldosari, Shakil A. Saghir, Mariam Cheema, Tahani Momenah, Kazim Husain, Yadollah Omidi, Syed A.A. Rizvi, Muhammad Akram, Rais A. Ansari
Toxicology Reports.2022; 9: 521. CrossRef - Potentiation of methylmercury toxicity by combined metal exposure: In vitro and in vivo models of a restricted metal exposome
Masahiro Akiyama, Yasuhiro Shinkai, Hiroto Yamakawa, Yun-Gi Kim, Yoshito Kumagai
Chemosphere.2022; 299: 134374. CrossRef - Application of 2,4,5‐tris(2‐pyridyl)imidazole as ‘turn‐off’ fluorescence sensor for Cu(II) and Hg(II) ions and in vitro cell imaging
Araghni Bhattacharya, Satyajit Mahata, Ashutosh Bandyopadhyay, Biman B. Mandal, Vadivelu Manivannan
Luminescence.2022; 37(6): 883. CrossRef - The Legacy of Mercury Contamination from a Past Leather Manufacturer and Health Risk Assessment in an Urban Area (Pisa Municipality, Italy)
Lisa Ghezzi, Simone Arrighi, Roberto Giannecchini, Monica Bini, Marta Valerio, Riccardo Petrini
Sustainability.2022; 14(7): 4367. CrossRef - Relationship between cumulative exposure to metal mixtures and heart rate among Chinese preschoolers
Ye Fu, Yun Liu, Yanli Liu, Yan Wang, Meiqin Zhu, Wei Lin, Mingzhu Li, Yang Liu, Minghui He, Lili Yu, Jing Wang
Chemosphere.2022; 300: 134548. CrossRef - The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review
Ying Tian, Jingjing Zhou, Changqing He, Lin He, Xingang Li, Hong Sui
Processes.2022; 10(4): 738. CrossRef - Characterisation of novel microbial strains Proteus mirabilis and Bordetella avium for heavy metal bioremediation and dye degradation
Rohini Ganorkar, Niti B. Jadeja, Arti Shanware, A. B. Ingle
Archives of Microbiology.2022;[Epub] CrossRef - Study on the elemental mercury removal performance of co-pyrolyzed Cl-loading activated carbon and the formation mechanism of C-Cl functional groups
Min Lv, Guangqian Luo, Renjie Zou, Qingyu Ji, Can Fang, Li Wang, Xian Li, Hong Yao
Fuel.2022; 322: 124229. CrossRef - Aqueous monomethylmercury degradation using nanoscale zero-valent iron through oxidative demethylation and reductive isolation
Ghulam Hussain Qasim, Hasan Fareed, Mijin Lee, Woojin Lee, Seunghee Han
Journal of Hazardous Materials.2022; 435: 128990. CrossRef - Understanding the Electrophoretic Deposition Accompanied by Electrochemical Reactions Toward Structurally Colored Bilayer Films
Naoki Tarutani, Ryo Uesugi, Kensuke Uemura, Kiyofumi Katagiri, Kei Inumaru, Yukikazu Takeoka
ACS Applied Materials & Interfaces.2022; 14(20): 23653. CrossRef - Potential human health risks of mercury-contaminated cassavas – Preliminary studies
Sylvester Addai-Arhin, Randy Novirsa, Hui Ho Jeong, Quang Dinh Phan, Nana Hirota, Yasuhiro Ishibashi, Hideki Shiratsuchi, Koji Arizono
Fundamental Toxicological Sciences.2022; 9(2): 61. CrossRef - Mercury maternal transfer in two placental sharks and a yolk-sac ray from Baja California Sur, Mexico
Isis Baró-Camarasa, Ana J. Marmolejo-Rodríguez, Todd M. O'Hara, J. Margaret Castellini, Daniela A. Murillo-Cisneros, Raúl O. Martínez-Rincón, Fernando R. Elorriaga-Verplancken, Felipe Galván-Magaña
Marine Pollution Bulletin.2022; 179: 113672. CrossRef - Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan
Muhammad Afzaal, Saman Hameed, Iram Liaqat, Amir Amanat Ali Khan, Hafiz abdul Manan, Raja Shahid, Muhammad Altaf
Water Practice and Technology.2022; 17(5): 1253. CrossRef - Contentious environmental governance in polluted gold mining geographies: The case of La Toma, Colombia
Irene Vélez-Torres, Diana Vanegas
World Development.2022; 157: 105953. CrossRef - The Concentration of Heavy Metals Cd, Hg, Pb in Processed Food Products Based Pterygoplichthys pardalis (Castelnau, 1855) from Ciliwung River Jakarta Region
Dewi Elfidasari, Haninah Haninah, Handhini Dwi Putri, Irawan Sugoro
BIOEDUSCIENCE.2022; 6(1): 73. CrossRef - Biosorption of heavy metals from water: mechanism, critical evaluation and translatability of methodology
Risha Jasmine Nathan, Arvind Kumar Jain, Rhonda J. Rosengren
Environmental Technology Reviews.2022; 11(1): 91. CrossRef - Higher mercury contamination is associated with shorter telomeres in a long-lived seabird – A direct effect or a consequence of among-individual variation in phenotypic quality?
Christina Bauch, Marie Claire Gatt, Simon Verhulst, José Pedro Granadeiro, Paulo Catry
Science of The Total Environment.2022; 839: 156359. CrossRef - Mercury levels in hair are associated with reduced neurobehavioral performance and altered brain structures in young adults
Hikaru Takeuchi, Yuka Shiota, Ken Yaoi, Yasuyuki Taki, Rui Nouchi, Ryoichi Yokoyama, Yuka Kotozaki, Seishu Nakagawa, Atsushi Sekiguchi, Kunio Iizuka, Sugiko Hanawa, Tsuyoshi Araki, Carlos Makoto Miyauchi, Kohei Sakaki, Takayuki Nozawa, Shigeyuki Ikeda, Su
Communications Biology.2022;[Epub] CrossRef - In vitro and in silico Studies Reveal Bacillus cereus AA-18 as a Potential Candidate for Bioremediation of Mercury-Contaminated Wastewater
Aatif Amin, Muhammad Naveed, Arslan Sarwar, Sunbul Rasheed, Hafiz Ghulam Murtaza Saleem, Zakia Latif, Andreas Bechthold
Frontiers in Microbiology.2022;[Epub] CrossRef - Oxygen as an important factor modulating in vitro MeHgCl toxicity associated with mitochondrial genes in hiPSCs
J. Augustyniak, G. Lipka, H. Kozlowska, F. Caloni, L. Buzanska
Ecotoxicology and Environmental Safety.2022; 241: 113737. CrossRef - Colorimetric sensing of mercury ions using green synthesized silver nanoparticles from Trigonella foenum (Linnaeus)
J. Infant, Thaninayagam Ebenezer, Gopi R.R., H. Joy Prabu, I. Johnson, Allen Joseph Anthuvan
Materials Today: Proceedings.2022; 68: 319. CrossRef - Influence of environmental and dietary exposures on metals accumulation among the residents of a major industrial harbour (Fos-sur-Mer, France)
Maxime Jeanjean, Sylvaine Goix, Julien Dron, Marine Periot, Annabelle Austruy, Khaled Douib, Renaud Persoons, Marie-Pierre Etienne, Gautier Revenko, Philippe Chamaret
Journal of Trace Elements in Medicine and Biology.2022; 73: 127021. CrossRef - Ferroptosis as a mechanism of non-ferrous metal toxicity
Michael Aschner, Anatoly V. Skalny, Airton C. Martins, Anton I. Sinitskii, Marcelo Farina, Rongzhu Lu, Fernando Barbosa, Yordanka G. Gluhcheva, Abel Santamaria, Alexey A. Tinkov
Archives of Toxicology.2022; 96(9): 2391. CrossRef - High Heat Resistance of the Structural Coloration of Colloidal Arrays with Inorganic Black Additives
Takahiro Yamanaka, Naoki Tarutani, Kiyofumi Katagiri, Kei Inumaru, Yukikazu Takeoka, Toshiyuki Masui
ACS Applied Materials & Interfaces.2022; 14(25): 29324. CrossRef - The health risk assessment of heavy metals to human health through the consumption of Tilapia spp and catfish caught from Lake Mariut, Egypt
Soha S. Hasanein, Mohamed H. Mourad, Afaf Mohamed M. Haredi
Heliyon.2022; 8(7): e09807. CrossRef - Potential Application of Living Microorganisms in the Detoxification of Heavy Metals
Runqiu Chen, Huaijun Tu, Tingtao Chen
Foods.2022; 11(13): 1905. CrossRef - Stress responses in captive Crocodylus moreletii associated with metal exposure
A.G. Romero-Calderón, T. Alvarez-Legorreta, J. Rendón von Osten, M. González-Jáuregui, J.R. Cedeño-Vázquez
Environmental Pollution.2022; 308: 119685. CrossRef - Age, body size, growth and dietary habits: What are the key factors driving individual variability in mercury of lacustrine fishes in northern temperate lakes?
Thomas A. Johnston, Gretchen L. Lescord, Michelle Quesnel, Pascale-Laure Savage, John M. Gunn, Karen A. Kidd
Environmental Research.2022; 213: 113740. CrossRef - Sulfate induced surface modification of Chlorella for enhanced mercury immobilization
Zhixin Wang, Zijia Zhang, Ling Xia, María Eugenia Farías, Rosa María Torres Sánchez, Carolina Belfiore, Maria Luciana Montes, Xiang Tian, Jinhui Chen, Shaoxian Song
Journal of Environmental Chemical Engineering.2022; 10(4): 108156. CrossRef - Effect of body size, feeding ecology and maternal transfer on mercury accumulation of vulnerable silky shark Carcharhinus falciformis in the eastern tropical pacific
Zezheng Li, Heidi R. Pethybridge, Yi Gong, Feng Wu, Xiaojie Dai, Yunkai Li
Environmental Pollution.2022; 309: 119751. CrossRef - Gaseous Mercury Exchange from Water–Air Interface in Differently Impacted Freshwater Environments
Federico Floreani, Alessandro Acquavita, Nicolò Barago, Katja Klun, Jadran Faganeli, Stefano Covelli
International Journal of Environmental Research and Public Health.2022; 19(13): 8149. CrossRef - Ultrasensitive colorimetric detection of Hg2+ based on glutathione-modified Au nanoflowers
Dehong Bai, Ziyu Xue, Peiqing Guo, Mingzhu Qiu, Xuefang Lei, Yujin Li, Chengjie Ma, Dongxia Zhang, Xibin Zhou
Microchemical Journal.2022; 181: 107766. CrossRef - Intracellular accumulation and DNA damage caused by methylmercury in glial cells
Aline H. Sousa, João P. G. Pereira, Allan C. Malaquias, Fernanda do E. S. Sagica, Edivaldo H. C. de Oliveira
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Whalers in “A Post-Whaling World”: Sustainable Conservation of Marine Mammals and Sustainable Development of Whaling Communities—With a Case Study from the Eastern Caribbean
Russell Fielding
Sustainability.2022; 14(14): 8782. CrossRef - Recent advances in nanomaterial developments for efficient removal of Hg(II) from water
Lata Rani, Arun Lal Srivastav, Jyotsna Kaushal, Xuan Cuong Nguyen
Environmental Science and Pollution Research.2022; 29(42): 62851. CrossRef - Evidence against Rapid Mercury Oxidation in Photochemical Smog
Seth N. Lyman, Tyler Elgiar, Mae Sexauer Gustin, Sarrah M. Dunham-Cheatham, Liji M. David, Lei Zhang
Environmental Science & Technology.2022; 56(16): 11225. CrossRef - Selective Sensing and Removal of Mercury Ions by Encapsulating Dansyl Appended Calix[4]Conjugate in a Zeolitic Imidazolate Framework as an Organic–Inorganic Hybrid Nanomaterial
Bhawna Uttam, Sirilata Polepalli, Shatarupa Sinha, Abhijit Majumder, Chebrolu Pulla Rao
ACS Applied Nano Materials.2022; 5(8): 11371. CrossRef - RETRACTED: Carveol ameliorates mercury-induced oxidative stress, neuroinflammation, and neurodegeneration in a mouse brain
Abdullah Alattar, Arooj Mohsin Alvi, Sajid Rashid, Nadia Hussain, Mehreen Gul, Muhammad Ikram, Atif Ali Khan Khalil, Reem Alshaman, Fawad Ali Shah, Shupeng Li, Jingbo Li
NeuroToxicology.2022; 92: 212. CrossRef - Novel Dynamic Technique, Nano-DIHM, for Rapid Detection of Oil, Heavy Metals, and Biological Spills in Aquatic Systems
Ryan Hall, Devendra Pal, Parisa A. Ariya
Analytical Chemistry.2022; 94(32): 11390. CrossRef - Plant and microbe mediated bioremediation: A long-term remedy for heavy metal pollution
Heena Bisht, Narayan Kumar
Asia Pacific Journal of Molecular Biology and Biotechnology.2022; : 69. CrossRef - Low-Level Environmental Mercury Exposure and Thyroid Cancer Risk Among Residents Living Near National Industrial Complexes in South Korea: A Population-Based Cohort Study
Seyoung Kim, Sang-Hwan Song, Chul-Woo Lee, Jung-Taek Kwon, Eun Young Park, Jin-Kyoung Oh, Hyun-Jin Kim, Eunjung Park, Byungmi Kim
Thyroid.2022; 32(9): 1118. CrossRef - Mercury Ion Binding to Apolipoprotein E Variants ApoE2, ApoE3, and ApoE4: Similar Binding Affinities but Different Structure Induction Effects
Elina Berntsson, Merlin Sardis, Andra Noormägi, Jüri Jarvet, Per M. Roos, Vello Tõugu, Astrid Gräslund, Peep Palumaa, Sebastian K. T. S. Wärmländer
ACS Omega.2022; 7(33): 28924. CrossRef - Recent progress on sustainable phytoremediation of heavy metals from soil
Mahdi Pouresmaieli, Mohammad Ataei, Pegah Forouzandeh, Paridokht Azizollahi, Matin Mahmoudifard
Journal of Environmental Chemical Engineering.2022; 10(5): 108482. CrossRef - Detecting mercury ions in water using a low-cost colorimetric sensor derived from immobilized silver nanoparticles on a paper substrate
Marco Laurence Budlayan, Jhonnybert Dalagan, Jeanne Phyre Lagare-Oracion, Jonathan Patricio, Susan Arco, Felmer Latayada, Temmy Vales, Benito Baje, Arnold Alguno, Rey Capangpangan
Environmental Nanotechnology, Monitoring & Management.2022; 18: 100736. CrossRef - Impairment in Working Memory and Executive Function Associated with Mercury Exposure in Indigenous Populations in Upper Amazonian Peru
Alycia K. Silman, Raveena Chhabria, George W. Hafzalla, Leahanne Giffin, Kimberly Kucharski, Katherine Myers, Carlos Culquichicón, Stephanie Montero, Andres G. Lescano, Claudia M. Vega, Luis E. Fernandez, Miles R. Silman, Michael J. Kane, John W. Sanders
International Journal of Environmental Research and Public Health.2022; 19(17): 10989. CrossRef - Mercury Exposure and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis
Behnam Ghorbani Nejad, Tahereh Raeisi, Parisa Janmohammadi, Fatemeh Mehravar, Mahtab Zarei, Azadeh Dehghani, Niki Bahrampour, Mohammad Hosein Darijani, Fatemeh Ahmadipour, Mohammad Mohajeri, Shahab Alizadeh, Harry H. X. Wang
International Journal of Clinical Practice.2022; 2022: 1. CrossRef - Organic Molecules Containing N, S and O Heteroatoms as Sensors for the Detection of Hg(II) Ion; Coordination and Efficiency toward Detection
Zarif Gul, Sikandar Khan, Ezzat Khan
Critical Reviews in Analytical Chemistry.2022; : 1. CrossRef - Fluorescent RET-Based Chemosensor Bearing 1,8-Naphthalimide and Styrylpyridine Chromophores for Ratiometric Detection of Hg2+ and Its Bio-Application
Pavel Panchenko, Anastasija Efremenko, Anna Polyakova, Alexey Feofanov, Maria Ustimova, Yuri Fedorov, Olga Fedorova
Biosensors.2022; 12(9): 770. CrossRef - The Association of Mercury and ALT with Obesity in Korean Adults: Using Data from the Korea National Health and Nutrition Examination Survey for 11 Years (KNHANES 2005, 2008~2017)
Sang Shin Pyo
Korean Journal of Clinical Laboratory Science.2022; 54(3): 192. CrossRef - The role of mercury and arsenic in the etiology and pathogenesis of Parkinson’s and Alzheimer’s diseases
V.N. Salkov, D.N. Voronkov, R.M. Khudoerkov
Arkhiv patologii.2022; 84(5): 59. CrossRef - Comparative study of Hg(ii) biosorption performance of xanthated and charred sugarcane bagasse from aqueous solutions
Puspa Lal Homagai, Mahesh Bhattarai, K. M. Radhika, Kedar Nath Ghimire, Hari Paudyal, Ajaya Bhattarai
RSC Advances.2022; 12(46): 29865. CrossRef - Carcinogenic effects of heavy metals by inducing dysregulation of microRNAs: A review
Amir Hossein Aalami, Mohammadsaleh Hoseinzadeh, Parsa Hosseini Manesh, Ali Jiryai Sharahi, Ehsan Kargar Aliabadi
Molecular Biology Reports.2022; 49(12): 12227. CrossRef - Concentration of mercury in human hair and associated factors in residents of the Gulf of Trieste (North-Eastern Italy)
Luca Cegolon, Elisa Petranich, Elena Pavoni, Federico Floreani, Nicolò Barago, Elisa Papassissa, Francesca Larese Filon, Stefano Covelli
Environmental Science and Pollution Research.2022; 30(8): 21425. CrossRef - Comprehending the Role of Endocrine Disruptors in Inducing Epigenetic
Toxicity
Arikath Kirtana, Barathi Seetharaman
Endocrine, Metabolic & Immune Disorders - Drug Targets.2022; 22(11): 1059. CrossRef - Mercury in Kansas Fish: Levels, Patterns, and Risk-Based Safe Consumption Limits for Mercury Sensitive Individuals
Clint A. Goodrich, Britini Jacobs, Brett T. Miller
Transactions of the Kansas Academy of Science.2022;[Epub] CrossRef - Exposure to mercury and thyroid function: Is there a connection?
Đurđica Marić, Vera Bonderović, Dragana Javorac, Katarina Baralić, Zorica Bulat, Danijela Đukić-Ćosić, Stefan Mandić-Rajčević, Miloš Žarković, Aleksandra Buha-Đorđević
Arhiv za farmaciju.2022; 72(5): 468. CrossRef - Effects of Low-Level Organic Mercury Exposure on Oxidative Stress Profile
Radu Ciprian Tincu, Cristian Cobilinschi, Iulia Alexandra Florea, Ana-Maria Cotae, Alexandru Emil Băetu, Sebastian Isac, Raluca Ungureanu, Gabriela Droc, Ioana Marina Grintescu, Liliana Mirea
Processes.2022; 10(11): 2388. CrossRef - Toxic metal and metalloid contamination in seafood from an eutrophic Brazilian estuary and associated public health risks
Paloma de Almeida Rodrigues, Rafaela Gomes Ferrari, Denes Kaic Alves do Rosário, Cristine Couto de Almeida, Tatiana Dillenburg Saint'Pierre, Rachel Ann Hauser-Davis, Luciano Neves dos Santos, Carlos Adam Conte-Junior
Marine Pollution Bulletin.2022; 185: 114367. CrossRef - Mercury Contamination in Fish and Its Effects on the Health of Pregnant Women and Their Fetuses, and Guidance for Fish Consumption—A Narrative Review
Bojian Chen, Shiyuan Dong
International Journal of Environmental Research and Public Health.2022; 19(23): 15929. CrossRef - Health risk assessment of heavy metals (Zn, Pb, Cd, and Hg) in water and muscle tissue of farmed carp species in North Iran
Mohammad Ali Hosseinzadeh Aski, Shayan Ghobadi, Abolfazl Askari Sari, Rashid Alijani Ardeshir, Mohammad Hossein Gorjian Arabi, Hamed Manouchehri
Environmental Science and Pollution Research.2022; 30(12): 32464. CrossRef - Advances in electrospun nanofibrous membrane sensors for ion detection
Liangqiang Wu, Yan Song, Shuo Xing, Yapeng Li, Hai Xu, Qingbiao Yang, Yaoxian Li
RSC Advances.2022; 12(54): 34866. CrossRef - Clinical and inflammatory biomarkers of inflammatory bowel diseases are linked to plasma trace elements and toxic metals; new insights into an old concept
Charalampia Amerikanou, Sotirios Karavoltsos, Aristea Gioxari, Dimitra Tagkouli, Aikaterini Sakellari, Efstathia Papada, Nick Kalogeropoulos, Alastair Forbes, Andriana C. Kaliora
Frontiers in Nutrition.2022;[Epub] CrossRef - Cell volume restriction by mercury chloride reduces M1-like inflammatory response of bone marrow-derived macrophages
Yen-Chieh Chuang, Shu-Yu Wu, Yu-Chuan Huang, Chung-Kan Peng, Shih-En Tang, Kun-Lun Huang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress
Sarita Pyatha, Haesoo Kim, Daeun Lee, Kisok Kim
Antioxidants.2022; 11(12): 2467. CrossRef - Exergo-economic and exergo-environmental analysis of a binary geothermal power plant with solar boosting
E. Giusti, L. Ciappi, P. Ungar, C. Zuffi, D. Fiaschi, G. Manfrida, L. Talluri
Journal of Physics: Conference Series.2022; 2385(1): 012124. CrossRef - Self-Associated 1,8-Naphthalimide as a Selective Fluorescent Chemosensor for Detection of High pH in Aqueous Solutions and Their Hg2+ Contamination
Awad I. Said, Desislava Staneva, Silvia Angelova, Ivo Grabchev
Sensors.2022; 23(1): 399. CrossRef - Health Risk Assessment for Human Exposure to Heavy Metals via Food Consumption in Inhabitants of Middle Basin of the Atrato River in the Colombian Pacific
Gabriel Caicedo-Rivas, Manuel Salas-Moreno, José Marrugo-Negrete
International Journal of Environmental Research and Public Health.2022; 20(1): 435. CrossRef - Evaluation of the concentration of heavy metals in vegetables from Ecuador
Ámbar Benavides, Braulio Romero, Iris Pérez-Almeida, Beatriz Pernía
Bionatura.2022; 7(3): 1. CrossRef -
Bioremediation of mercury-polluted soil and water by the plant symbiotic fungus
Metarhizium robertsii
Congcong Wu, Dan Tang, Jin Dai, Xingyuan Tang, Yuting Bao, Jiali Ning, Qing Zhen, Hui Song, Raymond J. St. Leger, Weiguo Fang
Proceedings of the National Academy of Sciences.2022;[Epub] CrossRef - Evaluation of chelating and cytoprotective activity of vanillin against the toxic action of mercuric chloride as an alternative for phytoremediation
Joelma P. da Silva, Maria do S. Costa, Fabia F. Campina, Camila F. Bezerra, Thiago S. de Freitas, Amanda K. Sousa, Celestina E. Sobral Souza, Yedda M. L. S. de Matos, Francisco N. Pereira-Junior, Irwim R. A. Menezes, Henrique D. M. Coutinho, Janaína E. Ro
Environmental Geochemistry and Health.2021; 43(4): 1609. CrossRef - Association between mercury in cord serum and sex-specific DNA methylation in cord tissues
Shino Nishizawa-Jotaki, Kenichi Sakurai, Akifumi Eguchi, Hiromi Tanabe, Masahiro Watanabe, Chisato Mori
Journal of Developmental Origins of Health and Disease.2021; 12(1): 124. CrossRef - Synthesis of a new thiophenol-thiophene polymer for the removal of mercury from wastewater and liquid hydrocarbons
Muhammad Alasad Albakri, Tawfik A. Saleh, Youcef Mankour, Thomas F. Garrison, Othman Charles S. Al Hamouz
Journal of Colloid and Interface Science.2021; 582: 428. CrossRef - Impact of Methylmercury and Other Heavy Metals Exposure on Neurocognitive Function in Children Aged 7 Years: Study Protocol of the Follow-up
Liza Vecchi Brumatti, Valentina Rosolen, Marika Mariuz, Elisa Piscianz, Erica Valencic, Maura Bin, Emmanouil Athanasakis, Pio D’Adamo, Eirini Fragkiadoulaki, Gemma Calamandrei, Öykü Dinckol, Fabio Barbone, Luca Ronfani
Journal of Epidemiology.2021; 31(2): 157. CrossRef - Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice
Yulan Zhao, Changming Zhou, Xiaoquan Guo, Guoliang Hu, Guyue Li, Yu Zhuang, Huabin Cao, Lin Li, Chonghong Xing, Caiying Zhang, Fan Yang, Ping Liu
Biological Trace Element Research.2021; 199(5): 1900. CrossRef - AIE-based fluorescent sensors for low concentration toxic ion detection in water
Haibo Wan, Qingfeng Xu, Peiyang Gu, Hua Li, Dongyun Chen, Najun Li, Jinghui He, Jianmei Lu
Journal of Hazardous Materials.2021; 403: 123656. CrossRef - First record on mercury accumulation in mice brain living in active volcanic environments: a cytochemical approach
A. Navarro-Sempere, Y. Segovia, A. S. Rodrigues, P. V. Garcia, R. Camarinho, M. García
Environmental Geochemistry and Health.2021; 43(1): 171. CrossRef - Heavy Metal Concentrations in Trachurus Mediterraneus and Merlangius Merlangus Captured from Marmara Sea, Turkey and Associated Health Risks
Latife Köker, Fatih Aydın, Özcan Gaygusuz, Reyhan Akçaalan, Derya Çamur, Hüseyin İlter, Ferruh Niyazi Ayoğlu, Ahmet Altın, Murat Topbaş, Meriç Albay
Environmental Management.2021; 67(3): 522. CrossRef - Trends and biological effects of environmental contaminants in lamprey
Charles P. Madenjian, Julia R. Unrein, Sílvia Pedro
Journal of Great Lakes Research.2021; 47: S112. CrossRef - Methylmercury-Induced Toxicopathologic Findings in Salivary Glands of Offspring Rats After Gestational and Lactational Exposure
Priscila Cunha Nascimento, Maria Karolina Martins Ferreira, Karolyny Martins Balbinot, Sérgio Melo Alves-Júnior, João de Jesus Viana Pinheiro, Felipe Martins Silveira, Manoela Domingues Martins, Maria Elena Crespo-Lopez, Rafael Rodrigues Lima
Biological Trace Element Research.2021; 199(8): 2983. CrossRef - Accumulation of Trace Metals in Indigenous Fish Species from the Old Brahmaputra River in Bangladesh and Human Health Risk Implications
Sabikunnahar Shorna, Saika Shawkat, Anwar Hossain, Shamshad B. Quraishi, A. K. M. Atique Ullah, Mohammad Mozammal Hosen, Md. Kamal Hossain, Badhan Saha, Bijoya Paul, Md. Habibullah-Al-Mamun
Biological Trace Element Research.2021; 199(9): 3478. CrossRef - A potential route for photolytic reduction of HgCl2 and HgBr2 in dry air and analysis about the impacts from Ozone
Yindong Tong, Hefeng Zhang, Huiming Lin, Benjamin de Foy, Long Chen, Wei Zhang, Xuejun Wang, Chunfeng Guan
Atmospheric Research.2021; 249: 105310. CrossRef - A novel dehydroabietic acid-based fluorescent probe for detection of Fe3+ and Hg2+ ions and its application in live-cell imaging
A-Liang Li, Zhong-Long Wang, Wen-Yan Wang, Qing-Song Liu, Yue Sun, Shi-Fa Wang, Wen Gu
Microchemical Journal.2021; 160: 105682. CrossRef - Colorimetric detection of Hg2+ and CH3Hg+ by a novel spirooxazine derivative as a highly sensitive and selective probe
Supak Pattaweepaiboon, Tanin Nanok, Narongpol Kaewchangwat, Khomson Suttisintong, Weekit Sirisaksoontorn
Dyes and Pigments.2021; 186: 108996. CrossRef - Nanomolar detection of mercury(II) using electropolymerized phthalocyanine film
Manjunatha Palanna, Shambhulinga Aralekallu, CP Keshavananda Prabhu, Veeresh A Sajjan, Mounesh, Lokesh Koodlur Sannegowda
Electrochimica Acta.2021; 367: 137519. CrossRef - A comparison of two bidirectional air-surface exchange models for gaseous elemental mercury over vegetated surfaces
Jingliang Hao, Xiaohong Xu, Che-Jen Lin, Leiming Zhang
Atmospheric Environment.2021; 246: 118096. CrossRef - Arsenic and mercury uptake and accumulation in oilseed sunflower accessions selected to mitigate co-contaminated soil coupled with oil and bioenergy production
Zulfiqar Ali Sahito, Afsheen Zehra, Lin Tang, Zarina Ali, Muhammad Laeeq ur Rehman Hashmi, Nabila Bano, Muhammad Asmat Ullah, Zhenli He, Xiaoe Yang
Journal of Cleaner Production.2021; 291: 125226. CrossRef - Carbon Nanodots in Electrochemical Sensors and Biosensors: A Review
Zahra Hassanvand, Fahimeh Jalali, Maryam Nazari, Fatemeh Parnianchi, Carlo Santoro
ChemElectroChem.2021; 8(1): 15. CrossRef - Use of mercury isotopes to quantify sources of human inorganic mercury exposure and metabolic processes in the human body
Buyun Du, Runsheng Yin, Xuewu Fu, Ping Li, Xinbin Feng, Laurence Maurice
Environment International.2021; 147: 106336. CrossRef - Inorganic mercury effects on biomarker gene expressions of a freshwater amphipod at two temperatures
Madson Silveira de Melo, Krishna Das, Eric Gismondi
Ecotoxicology and Environmental Safety.2021; 209: 111815. CrossRef - Modeling exposure risk and prevention of mercury in drinking water for artisanal-small scale gold mining communities
V. L. Morgan, L. Casso-Hartmann, I. Velez-Torres, D. C. Vanegas, R. Muñoz-Carpena, E. S. McLamore, G. A. Kiker
Human and Ecological Risk Assessment: An International Journal.2021; 27(6): 1492. CrossRef - Role of metallic pollutants in neurodegeneration: effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder
Ishnoor Kaur, Tapan Behl, Lotfi Aleya, Md. Habibur Rahman, Arun Kumar, Sandeep Arora, Rokeya Akter
Environmental Science and Pollution Research.2021; 28(8): 8989. CrossRef - Collapsed mitochondrial cristae in goat spermatozoa due to mercury result in lethality and compromised motility along with altered kinematic patterns
Bhawna Kushawaha, Rajkumar Singh Yadav, Dilip Kumar Swain, Priyambada Kumari, Akhilesh Kumar, Brijesh Yadav, Mukul Anand, Sarvajeet Yadav, Dipty Singh, Satish Kumar Garg
Scientific Reports.2021;[Epub] CrossRef - Mercury in neonatal and juvenile blacktip sharks (Carcharhinus limbatus). Part II: Effects assessment
Sarah B. Norris, Nicole A. Reistad, Darren G. Rumbold
Ecotoxicology.2021; 30(2): 311. CrossRef - Heavy metal- and organic-matter pollution due to self-heating coal-waste dumps in the Upper Silesian Coal Basin (Poland)
Ádám Nádudvari, Barbara Kozielska, Anna Abramowicz, Monika Fabiańska, Justyna Ciesielczuk, Jerzy Cabała, Tomasz Krzykawski
Journal of Hazardous Materials.2021; 412: 125244. CrossRef - Underlying mechanisms of cytotoxicity in HepG2 hepatocarcinoma cells exposed to arsenic, cadmium and mercury individually and in combination
W. Cordier, M. Yousaf, M.J. Nell, V. Steenkamp
Toxicology in Vitro.2021; 72: 105101. CrossRef - Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding
Shanjun Song, Yiling Li, Qian S. Liu, Huiyu Wang, Penghui Li, Jianbo Shi, Ligang Hu, Haiyan Zhang, Yuanchen Liu, Kun Li, Xingchen Zhao, Zongwei Cai
Journal of Hazardous Materials.2021; 412: 125158. CrossRef - Bis-BODIPY linked-triazole based on catechol core for selective dual detection of Ag+ and Hg2+
Worakrit Saiyasombat, Supavadee Kiatisevi
RSC Advances.2021; 11(6): 3703. CrossRef - A dual colorimetric probe for rapid environmental monitoring of Hg2+ and As3+ using gold nanoparticles functionalized with d-penicillamine
Su-Jin Yoon, Yun-Sik Nam, Yeonhee Lee, In Hwan Oh, Kang-Bong Lee
RSC Advances.2021; 11(10): 5456. CrossRef - Speciation analysis in chemistry
Waldo Quiroz
ChemTexts.2021;[Epub] CrossRef - Plasmonic Detection of Mercury via Amalgamation on Gold Nanorods Coated with PEG-Thiol
John R. Crockett, Hla Win-Piazza, Joseph E. Doebler, Tianqi Luan, Ying Bao
ACS Applied Nano Materials.2021; 4(2): 1654. CrossRef - Selective Hg2+ sensor: rGO-blended PEDOT:PSS conducting polymer OFET
Pasha W. Sayyad, Nikesh N. Ingle, Theeazen Al-Gahouari, Manasi M. Mahadik, Gajanan A. Bodkhe, Sumedh M. Shirsat, Mahendra D. Shirsat
Applied Physics A.2021;[Epub] CrossRef - Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium
Armin Salek Maghsoudi, Shokoufeh Hassani, Kayvan Mirnia, Mohammad Abdollahi
International Journal of Nanomedicine.2021; Volume 16: 803. CrossRef - Environmentally Benign Synthesis and Color Tuning of Strontium–Tantalum Perovskite Oxynitride and Its Solid Solutions
Takuya Sakata, Risa Yoshiyuki, Ryoki Okada, Sohta Urushidani, Naoki Tarutani, Kiyofumi Katagiri, Kei Inumaru, Kyohei Koyama, Yuji Masubuchi
Inorganic Chemistry.2021; 60(7): 4852. CrossRef - A scoping review of sources of mercury and its health effects among Pakistan’s most vulnerable population
Ejaz Ahmad Khan, Zaigham Abbas
Reviews on Environmental Health.2021; 36(1): 39. CrossRef - Total blood mercury and its determinants in New Zealand children and adults
Andrea ’t Mannetje, Jonathan Coakley, Jeroen Douwes
Journal of Exposure Science & Environmental Epidemiology.2021; 31(2): 289. CrossRef - Inkjet-printed paper-based colorimetric sensor coupled with smartphone for determination of mercury (Hg2+)
Monisha, Kamlesh Shrivas, Tushar Kant, Sanyukta Patel, Rama Devi, Nohar Singh Dahariya, Shamsh Pervez, Manas Kanti Deb, Manish K. Rai, Joyce Rai
Journal of Hazardous Materials.2021; 414: 125440. CrossRef - Machine learning-assisted calibration of Hg2+ sensors based on carbon nanotube field-effect transistors
Long Bian, Zhunheng Wang, David L. White, Alexander Star
Biosensors and Bioelectronics.2021; 180: 113085. CrossRef - Transcriptome Analysis Reveals HgCl2 Induces Apoptotic Cell Death in Human Lung Carcinoma H1299 Cells through Caspase-3-Independent Pathway
Mi Jin Kim, Jinhong Park, Jinho Kim, Ji-Young Kim, Mi-Jin An, Geun-Seup Shin, Hyun-Min Lee, Chul-Hong Kim, Jung-Woong Kim
International Journal of Molecular Sciences.2021; 22(4): 2006. CrossRef - Geothermal power plants with improved environmental performance: assessment of the potential for an Italian site
Daniele Fiaschi, Martina Leveni, Giampaolo Manfrida, Barbara Mendecka, Lorenzo Talluri, U. Desideri, L. Ferrari, J. Yan
E3S Web of Conferences.2021; 238: 01010. CrossRef - Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives
Emanuela D. Tiodar, Cristina L. Văcar, Dorina Podar
International Journal of Environmental Research and Public Health.2021; 18(5): 2435. CrossRef - Effects of mercury at field estimated concentration in brain of Bombus atratus (Hymenoptera: Bombini)
Michele Provase, Raquel Fernanda Salla, Cíntia Requião de Lima, Fábio Camargo Abdalla
Chemosphere.2021; 276: 130198. CrossRef - Time to refine mercury mass balance models for fish
Charles P. Madenjian, Steven R. Chipps, Paul J. Blanchfield, Mark Mallory
FACETS.2021; 6(1): 272. CrossRef - Distribution and bioavailability of mercury in the surface sediments of the Baltic Sea
Urszula Kwasigroch, Magdalena Bełdowska, Agnieszka Jędruch, Katarzyna Łukawska-Matuszewska
Environmental Science and Pollution Research.2021; 28(27): 35690. CrossRef - Functionalized Gold Nanoparticles as an Active Layer for Mercury Vapor Detection at Room Temperature
Ilaria Fratoddi, Sara Cerra, Tommaso A. Salamone, Raoul Fioravanti, Fabio Sciubba, Emiliano Zampetti, Antonella Macagnano, Amanda Generosi, Barbara Paci, Francesca A. Scaramuzzo, Roberto Matassa, Giuseppe Familiari, Chiara Battocchio, Martina Marsotto, Pa
ACS Applied Nano Materials.2021; 4(3): 2930. CrossRef - Mercury offloading in gametes and potential adverse effects of high mercury concentrations in blood and tissues of Atlantic Goliath Grouper Epinephelus itajara in the southeastern United States
Christopher R. Malinowski, Nicole I. Stacy, Felicia C. Coleman, Jessica A. Cusick, Carle M. Dugan, Christopher C. Koenig, Natassjia K. Ragbeer, Justin R. Perrault
Science of The Total Environment.2021; 779: 146437. CrossRef - The Prevalence of Inorganic Mercury in Human Kidneys Suggests a Role for Toxic Metals in Essential Hypertension
Roger Pamphlett, Philip A. Doble, David P. Bishop
Toxics.2021; 9(3): 67. CrossRef - Mercury in Blood of Children Exposed to Historical Residues from Metallurgical Activity
Ofelia Morton Bermea, Javier Castro-Larragoitia, Ángel Alberto Arellano Álvarez, Rebeca Jazmín Pérez-Rodríguez, Adriana Leura-Vicencio, Benedetto Schiavo, Elías Salgado-Martínez, Israel Razo Soto, Elizabeth Hernández Álvarez
Exposure and Health.2021; 13(2): 281. CrossRef - Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals
J.P. Desforges, B. Mikkelsen, M. Dam, F. Rigét, S. Sveegaard, C. Sonne, R. Dietz, N. Basu
NeuroToxicology.2021; 84: 136. CrossRef - Adverse cardiovascular effects of exposure to cadmium and mercury alone and in combination on the cardiac tissue and aorta of Sprague–Dawley rats
Sandra Arbi, Megan Jean Bester, Liselle Pretorius, Hester Magdalena Oberholzer
Journal of Environmental Science and Health, Part A.2021; 56(6): 609. CrossRef - Biochemical responses of oysters in evaluating environmental quality of tropical Indian estuarine systems
Prantick Patra, Chellandi Mohandass, Parthasarathi Chakraborty, Seyieleno C. Seleyi
Chemosphere.2021; 278: 130338. CrossRef - Cellular and Molecular Mechanisms Mediating Methylmercury Neurotoxicity and Neuroinflammation
João P. Novo, Beatriz Martins, Ramon S. Raposo, Frederico C. Pereira, Reinaldo B. Oriá, João O. Malva, Carlos Fontes-Ribeiro
International Journal of Molecular Sciences.2021; 22(6): 3101. CrossRef - A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal
M. Abhinaya, R. Parthiban, P. Senthil Kumar, Dai-Viet N. Vo
Environmental Research.2021; 196: 110996. CrossRef - Contribution of trace element exposure to gestational diabetes mellitus through disturbing the gut microbiome
Yuqing Zhang, Ting Chen, Yiyun Zhang, Qi Hu, Xu Wang, Hang Chang, Jian-Hua Mao, Antoine M. Snijders, Yankai Xia
Environment International.2021; 153: 106520. CrossRef - Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management
Khadga Raj, Pawandeep Kaur, G.D. Gupta, Shamsher Singh
Neuroscience Letters.2021; 753: 135873. CrossRef - Trends in sensor development toward next-generation point-of-care testing for mercury
Ji Won Lim, Tai-Yong Kim, Min-Ah Woo
Biosensors and Bioelectronics.2021; 183: 113228. CrossRef - Research Progress on the Detection Method of Mercury and Its Species in Water
健 赵
Advances in Environmental Protection.2021; 11(02): 243. CrossRef - Highly sensitive and selective colorimetric detection of dual metal ions (Hg2+ and Sn2+) in water: an eco-friendly approach
Rintumoni Paw, Moushumi Hazarika, Purna K. Boruah, Amlan Jyoti Kalita, Ankur K. Guha, Manash R. Das, Chandan Tamuly
RSC Advances.2021; 11(24): 14700. CrossRef - Macro and Trace Elements in Hemp (Cannabis sativa L.) Cultivated in Greece: Risk Assessment of Toxic Elements
Effrosyni Zafeiraki, Konstantinos M. Kasiotis, Paul Nisianakis, Kyriaki Machera
Frontiers in Chemistry.2021;[Epub] CrossRef - Chronic exposure to volcanic gaseous elemental mercury: using wild Mus musculus to unveil its uptake and fate
R. Camarinho, A. Navarro-Sempere, P. V. Garcia, M. García, Y. Segovia, A. S. Rodrigues
Environmental Geochemistry and Health.2021; 43(11): 4863. CrossRef - How do trophic magnification factors (TMFs) and biomagnification factors (BMFs) perform on toxic pollutant bioaccumulation estimation in coastal and marine food webs
Zhen Wang, Yue Li, Fanlong Kong, Minghui Li, Min Xi, Zhengda Yu
Regional Studies in Marine Science.2021; 44: 101797. CrossRef - Low-Cost Organic Adsorbents for Elemental Mercury Removal from Lignite Flue Gas
Marta Marczak-Grzesik, Stanisław Budzyń, Barbara Tora, Szymon Szufa, Krzysztof Kogut, Piotr Burmistrz
Energies.2021; 14(8): 2174. CrossRef - Effect of metal ions on the thermo-optic properties of Rhodamine 6G-gold nanoparticle hybrids
Jisha John, Achamma Kurian, Sajan D. George
Optik.2021; 241: 166988. CrossRef - Interactions between mercury and environmental factors: A chemometric assessment in seafood from an eutrophic estuary in southeastern Brazil
Paloma de Almeida Rodrigues, Rafaela Gomes Ferrari, Denes Kaic Alves do Rosário, Rachel Ann Hauser-Davis, Amanda Pontes Lopes, Alejandra Filippo Gonzalez Neves dos Santos, Carlos Adam Conte-Junior
Aquatic Toxicology.2021; 236: 105844. CrossRef - Cardiometabolic risk factors and mercury content in hair of women from a territory distant from mercury-rich geochemical zones (Cherepovets city, Northwest Russia)
E. S. Ivanova, O. P. Shuvalova, L. S. Eltsova, V. T. Komov, A. I. Kornilova
Environmental Geochemistry and Health.2021; 43(11): 4589. CrossRef - Mercury in Barents Sea fish in the Arctic polar night: Species and spatial comparison
Anjali Gopakumar, Julia Giebichenstein, Evgeniia Raskhozheva, Katrine Borgå
Marine Pollution Bulletin.2021; 169: 112501. CrossRef - Effects of Treadmill Exercise on the Expression Level of BAX, BAD, BCL-2, BCL-XL, TFAM, and PGC-1α in the Hippocampus of Thimerosal-Treated Rats
Pouria Navazani, Salar Vaseghi, Mehrdad Hashemi, Mohammad-Reza Shafaati, Mohammad Nasehi
Neurotoxicity Research.2021; 39(4): 1274. CrossRef - Wastewater Treatment and Reuse: a Review of its Applications and Health Implications
Kavindra Kumar Kesari, Ramendra Soni, Qazi Mohammad Sajid Jamal, Pooja Tripathi, Jonathan A. Lal, Niraj Kumar Jha, Mohammed Haris Siddiqui, Pradeep Kumar, Vijay Tripathi, Janne Ruokolainen
Water, Air, & Soil Pollution.2021;[Epub] CrossRef - A rapid “on-off-on” peptide-based fluorescent probe for selective and consecutive detection of mercury and sulfide ions in aqueous systems and live cells
Shaohua Wen, Qifan Wang, Zhouquan Guo, Bo Chen, Yi Liu, Peng Wang, Xiupei Yang, Yong An
Journal of Photochemistry and Photobiology A: Chemistry.2021; 417: 113354. CrossRef - Impact of environmental mercury exposure on the blood cells oxidative status of fishermen living around Mundaú lagoon in Maceió – Alagoas (AL), Brazil
Reginaldo Silva-Filho, Nerveson Santos, Mayara Costa Santos, Ábner Nunes, Raphael Pinto, Chiara Marinho, Talitta Lima, Mariana P. Fernandes, Josué Carinhanha C. Santos, Ana Catarina R. Leite
Ecotoxicology and Environmental Safety.2021; 219: 112337. CrossRef - Bioremediation: An effective approach of mercury removal from the aqueous solutions
Lata Rani, Arun Lal Srivastav, Jyotsna Kaushal
Chemosphere.2021; 280: 130654. CrossRef - Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis
Shweta Tripathi, Krishna Mohan Poluri
Environmental Pollution.2021; 285: 117443. CrossRef - Single and mixed exposure to cadmium and mercury in Drosophila melanogaster: Molecular responses and impact on post-embryonic development
Laëtitia Frat, Thomas Chertemps, Elise Pesce, Françoise Bozzolan, Matthieu Dacher, Rosario Planelló, Oscar Herrero, Lola Llorente, Didier Moers, David Siaussat
Ecotoxicology and Environmental Safety.2021; 220: 112377. CrossRef - Effects of arsenic and heavy metals on metabolic pathways in cells of human origin: Similarities and differences
Kaniz Fatema, Sabrina Samad Shoily, Tamim Ahsan, Zinia Haidar, Ahmed Faisal Sumit, Abu Ashfaqur Sajib
Toxicology Reports.2021; 8: 1109. CrossRef - Bee venom Apis mellifera lamarckii rescues blood brain barrier damage and neurobehavioral changes induced by methyl mercury via regulating tight junction proteins expression in rat cerebellum
Ehsan H. Abu-Zeid, Bouthaina A. Khalifa, Yaser H.A. Elewa, Ahmed H. Arisha, Tamer A. Ismail, Basma M. Hendam, Shereen El Abdel-Hamid
Food and Chemical Toxicology.2021; 154: 112309. CrossRef - The silver linings of mercury: Reconsideration of its impacts on living organisms from a multi-timescale perspective
Chengjun Li, Jun Shen, Jin Zhang, Pei Lei, Yaqi Kong, Jichao Zhang, Wenli Tang, Tianyu Chen, Xin Xiang, Shuxiao Wang, Wei Zhang, Huan Zhong
Environment International.2021; 155: 106670. CrossRef - Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils
Sandhya Mishra, Ziqiu Lin, Shimei Pang, Yuming Zhang, Pankaj Bhatt, Shaohua Chen
Journal of Hazardous Materials.2021; 418: 126253. CrossRef - Scalable approach towards specific and ultrasensitive cation sensing under harsh environmental conditions by engineering the analyte–transducer interface
Sudeshna Mondal, Chandramouli Subramaniam
Nanoscale Advances.2021; 3(13): 3752. CrossRef - Bioaccumulation of Toxic Metals in Children Exposed to Urban Pollution and to Cement Plant Emissions
Agostino Di Ciaula
Exposure and Health.2021; 13(4): 681. CrossRef - Levels of metals and persistent organic pollutants in traditional foods consumed by First Nations living on-reserve in Canada
Hing Man Chan, Kavita Singh, Malek Batal, Lesya Marushka, Constantine Tikhonov, Tonio Sadik, Harold Schwartz, Amy Ing, Karen Fediuk
Canadian Journal of Public Health.2021; 112(S1): 81. CrossRef - A Functionalised Carbon Fiber for Flexible Extraction and Determination of Hg(II) Using Au(NP)-Thiol-CF Inductively Coupled Plasma Mass Spectrometry
Mashael K. Bin Ateeq, Nouf M. Bin Durayhim, Meral M. Sulayem, Waad A. Al-Qahtani, Nezar H. Khdary, Ahmed M. Alhassan, Fatimah Mohammed A. Alzahrani, Khadijah Mohammedsaleh M. Katubi, Norah Salem Alsaiari
Water.2021; 13(13): 1829. CrossRef - Mercury distribution in the East Himalayas: Elevational patterns in soils and non-volant small mammals
Yanju Ma, Lihai Shang, Huijian Hu, Wei Zhang, Lianghua Chen, Zhixin Zhou, Paras Bikram Singh, Yiming Hu
Environmental Pollution.2021; 288: 117752. CrossRef - Plasmon-Coupled Silver Nanoparticles for Mobile Phone-Based Attomolar Sensing of Mercury Ions
Sriram Rathnakumar, Seemesh Bhaskar, Aayush Rai, Darisi V. V. Saikumar, Naga Sai Visweswar Kambhampati, Venketesh Sivaramakrishnan, Sai Sathish Ramamurthy
ACS Applied Nano Materials.2021; 4(8): 8066. CrossRef - Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater
Pooja Sharma, Ashutosh Kumar Pandey, Sang-Hyoun Kim, Surendra Pratap Singh, Preeti Chaturvedi, Sunita Varjani
Environmental Technology & Innovation.2021; 24: 101826. CrossRef - Methyl mercury triggers endothelial leukocyte adhesion and increases expression of cell adhesion molecules and chemokines
Joshua Fowler, Martin Tsz-Ki Tsui, Jessica Chavez, Safeera Khan, Hassan Ahmed, Lena Smith, Zhenquan Jia
Experimental Biology and Medicine.2021; 246(23): 2522. CrossRef - Exchange Reactions Alter Molecular Speciation of Gaseous Oxidized Mercury
Na Mao, Alexei Khalizov
ACS Earth and Space Chemistry.2021; 5(8): 1842. CrossRef - Magnetic solid-phase extraction based on sulfur-functionalized magnetic metal-organic frameworks for the determination of methylmercury and inorganic mercury in water and fish samples
Dian-Bing Zhou, Ya-Bing Xiao, Fang Han, Ya-Ning Lv, Lei Ding, Wei Song, Yu-Xin Liu, Ping Zheng, Da Chen
Journal of Chromatography A.2021; 1654: 462465. CrossRef - Genetic Polymorphism of Delta Aminolevulinic Acid Dehydratase (ALAD) Gene and Symptoms of Chronic Mercury Exposure in Munduruku Indigenous Children within the Brazilian Amazon
Jamila Alessandra Perini, Mayara Calixto Silva, Ana Claudia Santiago de Vasconcellos, Paulo Victor Sousa Viana, Marcelo Oliveira Lima, Iracina Maura Jesus, Joseph William Kempton, Rogério Adas Ayres Oliveira, Sandra Souza Hacon, Paulo Cesar Basta
International Journal of Environmental Research and Public Health.2021; 18(16): 8746. CrossRef - Synthesis, characterization, theoretical study and biological activity studies of the mercury (II) complexes of 5‐methyl‐5‐(4‐nitrophenyl)‐hydantoin
Leila Vatannavaz, Seyyed Javad Sabounchei, Asieh Sedghi, Roya Karamian, Seyed Hamed Moazzami Farida, Nosrat Rahmani
Journal of the Chinese Chemical Society.2021; 68(11): 2140. CrossRef - Highly Sensitive Colorimetric Naked-Eye Detection of HgII Using a Sacrificial Metal–Organic Framework
Somayeh Tarasi, Ali Ramazani, Ali Morsali, Mao-Lin Hu
Inorganic Chemistry.2021; 60(17): 13588. CrossRef - A rhodamine based nanosensor platform for Hg2+ sensing in near–perfect aqueous medium: Smartphone, test strip and real sample applications
Sukriye Nihan Karuk Elmas, Sinan Dinckan, Fatma Nur Arslan, Duygu Aydin, Tahir Savran, Ibrahim Yilmaz
Journal of Photochemistry and Photobiology A: Chemistry.2021; 421: 113521. CrossRef - Effect of Sonochemical Treatment on Thermal Stability, Elemental Mercury (Hg0) Removal, and Regenerable Performance of Magnetic Tea Biochar
Adnan Raza Altaf, Haipeng Teng, Liu Gang, Yusuf G. Adewuyi, Maosheng Zheng
ACS Omega.2021; 6(37): 23913. CrossRef - Total gaseous mercury levels in the vicinity of the Central Mexico mountain mining zone and its dispersion area
Rocío García-Martínez, Gilberto Hernández-Silva, Rubén Pavia-Hernández, Benedetto Schiavo, Miguel Flores-Espinosa, Ann Wellens, Ricardo Torres-Jardon, Agustín Garcia-Reynoso, Amparo Martínez-Arroyo, Arturo Gavilán-García, Luis G.Ruíz-Suárez
Air Quality, Atmosphere & Health.2021; 14(12): 1953. CrossRef - DNAzyme-Based Biosensors: Immobilization Strategies, Applications, and Future Prospective
Shadman Khan, Brenda Burciu, Carlos D. M. Filipe, Yingfu Li, Kristen Dellinger, Tohid F. Didar
ACS Nano.2021; 15(9): 13943. CrossRef - Interaction of plastic particles with heavy metals and the resulting toxicological impacts: a review
Sukhendu Maity, Chayan Biswas, Sambuddha Banerjee, Rajkumar Guchhait, Madhuchhanda Adhikari, Ankit Chatterjee, Kousik Pramanick
Environmental Science and Pollution Research.2021; 28(43): 60291. CrossRef - Detection of heavy metal ions using meander gated GaN HEMT sensor
Shivanshu Mishra, Pharyanshu Kachhawa, Rajiv Ranjan Thakur, Amber Kumar Jain, Kuldip Singh, Nidhi Chaturvedi
Sensors and Actuators A: Physical.2021; 332: 113119. CrossRef - Sexual dimorphism in inorganic mercury toxicokinetics and the attendant lipotoxic and non-lipotoxic dyslipidemia in the rat
A.D. Wusu, O.O. Ogunrinola, O.K. Afolabi, E.O. Abam, D.O. Babayemi, O.A. Dosumu, O.B. Onunkwor, E.A. Balogun, O.O. Odukoya, O. Ademuyiwa
Biochemistry and Biophysics Reports.2021; 28: 101146. CrossRef - Trace metal concentrations in the abiotic and biotic components of River Rwizi ecosystem in western Uganda, and the risks to human health
Anthony Basooma, Lies Teunen, Nathan Semwanga, Lieven Bervoets
Heliyon.2021; 7(11): e08327. CrossRef - BMAA, Methylmercury, and Mechanisms of Neurodegeneration in Dolphins: A Natural Model of Toxin Exposure
David A. Davis, Susanna P. Garamszegi, Sandra Anne Banack, Patrick D. Dooley, Thomas M. Coyne, Dylan W. McLean, David S. Rotstein, Deborah C. Mash, Paul Alan Cox
Toxins.2021; 13(10): 697. CrossRef - Remediation strategies for heavy metals contaminated ecosystem: A review
Mahendra Kumar, Aparna Seth, Alak Kumar Singh, Manish Singh Rajput, Mohd Sikandar
Environmental and Sustainability Indicators.2021; 12: 100155. CrossRef - Porphyrin-infiltrated SiO2 inverse opal photonic crystal as fluorescence sensor for selective detection of trace mercury ion
Heng Li, Zhaohua Xu, Ning Sun
Optical Materials.2021; 122: 111696. CrossRef - Contributing to Understand the Crosstalk between Brain and Periphery in Methylmercury Intoxication: Neurotoxicity and Extracellular Vesicles
Gabriela de Paula Arrifano, Marcus Augusto-Oliveira, Megan Sealey-Bright, Jaezah Zainal, Luciana Imbiriba, Luanna Melo Pereira Fernandes, Cristiane Socorro Ferraz Maia, Daniel Anthony, Maria Elena Crespo-Lopez
International Journal of Molecular Sciences.2021; 22(19): 10855. CrossRef - Recent Advances on the Development of Chemosensors for the Detection of Mercury Toxicity: A Review
Shiva Prasad Kollur, Chandan Shivamallu, Shashanka K. Prasad, Ravindra Veerapur, Sharanagouda S. Patil, Charley A. Cull, Johann F. Coetzee, Raghavendra G. Amachawadi
Separations.2021; 8(10): 192. CrossRef - Mercury Content in Dietary Supplements From Poland Containing Ingredients of Plant Origin: A Safety Assessment
Anna Puścion-Jakubik, Anita Mielech, Dominika Abramiuk, Małgorzata Iwaniuk, Monika Grabia, Joanna Bielecka, Renata Markiewicz-Żukowska, Katarzyna Socha
Frontiers in Pharmacology.2021;[Epub] CrossRef - Occurrence of total mercury and methylmercury in rice: Exposure and health implications in Nepal
Le Wang, Jialiang Han, Hem Bahadur Katuwal, Pinhua Xia, Xiaohang Xu, Xinbin Feng, Guangle Qiu
Ecotoxicology and Environmental Safety.2021; 228: 113019. CrossRef - Methylmercury and Polycyclic Aromatic Hydrocarbons in Mediterranean Seafood: A Molecular Anthropological Perspective
Andrea De Giovanni, Cristina Giuliani, Mauro Marini, Donata Luiselli
Applied Sciences.2021; 11(23): 11179. CrossRef - Indigenous mercury-resistant bacteria isolated from contaminated soils around artisanal gold processing centers in Sukabumi, Indonesia
F Y Amandita, Efadeswarni, Idris, T Sulistiyani, A Kanti, I M Sudiana
IOP Conference Series: Earth and Environmental Science.2021; 909(1): 012009. CrossRef - Genotoxicity of Mercury and Its Derivatives Demonstrated In Vitro and In Vivo in Human Populations Studies. Systematic Review
Juana Sánchez-Alarcón, Mirta Milić, Lilia Patricia Bustamante-Montes, Keila Isaac-Olivé, Rafael Valencia-Quintana, Ninfa Ramírez-Durán
Toxics.2021; 9(12): 326. CrossRef - Characterization of amalgamation tailings from ASGM in Sekotong area, West Nusa Tenggara, Indonesia, and its potential added value
A Wahyudi, W Surono, I Rodliyah, H E Mamby
IOP Conference Series: Earth and Environmental Science.2021; 882(1): 012070. CrossRef - Fish Sellers Knowledge on Exposure of Mercury from Fish in the Kenjeran Beach Area, Surabaya
Trias Mahmudiono, Eurika Zebadia, Diah Indriani, Stefania Setyaningtyas
Open Access Macedonian Journal of Medical Sciences.2021; 9(E): 1549. CrossRef - Mushroom Quality Related with Various Substrates’ Bioaccumulation and Translocation of Heavy Metals
Siti Maryam Salamah Ab Rhaman, Laila Naher, Shafiquzzaman Siddiquee
Journal of Fungi.2021; 8(1): 42. CrossRef - Adsorption and Removal of Hazardous Metallic Elements Hg 0, Ni 0 and Pb 0: A DFT Study on g-C 3N 4 Monolayer Modified with Pt n (n=1 - 7) Clusters
Siying Zhong, Shao-Yi Wu, Gao-Jun Zhang, Jia-Xing Guo, Li Yan
SSRN Electronic Journal .2021;[Epub] CrossRef - Acute and Chronic Toxicity Study of Vatavidhvamsana Rasa, an Ayurvedic Herbomineral Formulation
S. Mahesh, Swapnil Y. Chaudhary, Mukeshkumar Nariya, B. J. Patgiri
Toxicology International.2021; : 421. CrossRef - The threat of global mercury pollution to bird migration: potential mechanisms and current evidence
Chad L. Seewagen
Ecotoxicology.2020; 29(8): 1254. CrossRef - Evaluation of health risks associated with trace metal exposure in water from the Barekese reservoir in Kumasi, Ghana
O. Akoto, E. Gyimah, Z. Zhan, H. Xu, C. Nimako
Human and Ecological Risk Assessment: An International Journal.2020; 26(4): 1134. CrossRef - 100 years of high GEM concentration in the Central Italian Herbarium and Tropical Herbarium Studies Centre (Florence, Italy)
Jacopo Cabassi, Valentina Rimondi, Zhang Yeqing, Antonella Vacca, Orlando Vaselli, Antonella Buccianti, Pilario Costagliola
Journal of Environmental Sciences.2020; 87: 377. CrossRef - Mercury affects uterine myogenic activity even without producing any apparent toxicity in rats: Involvement of calcium-signaling cascades
Swati Koli, Atul Prakash, Soumen Choudhury, Rajesh Mandil, Satish K. Garg
Journal of Trace Elements in Medicine and Biology.2020; 57: 40. CrossRef - Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health
Rubina Khanam, Anjani Kumar, A.K. Nayak, Md. Shahid, Rahul Tripathi, S. Vijayakumar, Debarati Bhaduri, Upendra Kumar, Sangita Mohanty, P. Panneerselvam, Dibyendu Chatterjee, B.S. Satapathy, H. Pathak
Science of The Total Environment.2020; 699: 134330. CrossRef - Mercury and selenium concentrations, and selenium:mercury molar ratios in small cetaceans taken off St. Vincent, West Indies
Meaghan A. McCormack, Russell Fielding, Jeremy J. Kiszka, Valeria Paz, Brian P. Jackson, Don R. Bergfelt, Jessica Dutton
Environmental Research.2020; 181: 108908. CrossRef - Metalloproteomic approach of mercury-binding proteins in liver and kidney tissues of Plagioscion squamosissimus (corvina) and Colossoma macropomum (tambaqui) from Amazon region: Possible identification of mercury contamination biomarkers
Alis Correia Bittarello, José Cavalcante Souza Vieira, Camila Pereira Braga, Izabela da Cunha Bataglioli, Grasieli de Oliveira, Leone Campos Rocha, Luiz Fabrício Zara, Marília Afonso Rabelo Buzalaf, Lincoln Carlos Silva de Oliveira, Jiri Adamec, Pedro de
Science of The Total Environment.2020; 711: 134547. CrossRef - Mercury Accumulation and Effects in the Brain of the Atlantic Sharpnose Shark (Rhizoprionodon terraenovae)
S. L. Ehnert-Russo, J. Gelsleichter
Archives of Environmental Contamination and Toxicology.2020; 78(2): 267. CrossRef - Macrobrachium amazonicum (Crustacea, Decapoda) Used to Biomonitor Mercury Contamination in Rivers
Brenda Natasha Souza Costa, Helena Pereira Almeida, Bárbara Carolina Pereira da Silva, Lucas Gallat de Figueiredo, Adriana Marques de Oliveira, Marcelo de Oliveira Lima
Archives of Environmental Contamination and Toxicology.2020; 78(2): 245. CrossRef - Comparison of Bacterial Community Structure and Diversity in Traditional Gold Mining Waste Disposal Site and Rice Field by Using a Metabarcoding Approach
Fatimawali, Billy Johnson Kepel, Maria Apriliani Gani, Trina Ekawati Tallei
International Journal of Microbiology.2020; 2020: 1. CrossRef - Recent developments in environmental mercury bioremediation and its toxicity: A review
Shivani Kumari, Amit, Rahul Jamwal, Neha Mishra, Dileep Kumar Singh
Environmental Nanotechnology, Monitoring & Management.2020; 13: 100283. CrossRef - The kinetics of mercury vaporization in soil during low-temperature thermal treatment
Min-oh Park, Moon-Hyun Kim, Yongseok Hong
Geoderma.2020; 363: 114150. CrossRef - Association of Blood Mercury Level with the Risk of Depression According to Fish Intake Level in the General Korean Population: Findings from the Korean National Health and Nutrition Examination Survey (KNHANES) 2008–2013
Kyung Won Kim, Sundara Raj Sreeja, Minji Kwon, Ye Lee Yu, Mi Kyung Kim
Nutrients.2020; 12(1): 189. CrossRef - A Facile Preparation of a New Water-Soluble Acridine Derivative and Application as a Turn-off Fluorescence Chemosensor for Selective Detection of Hg2+
Marcelo Carpes Nunes, Fabiane dos Santos Carlos, Otávio Fuganti, Letícia Aparecida da Silva, Hennrique Taborda Ribas, Sheila Maria Brochado Winnischofer, Fábio Souza Nunes
Journal of Fluorescence.2020; 30(2): 235. CrossRef - Mercury in fish marketed in the Amazon Triple Frontier and Health Risk Assessment
Stephani Ferreira da Silva, Marcelo de Oliveira Lima
Chemosphere.2020; 248: 125989. CrossRef - Canadian Arctic Contaminants and Their Effects on the Maternal Brain and Behaviour: A Scoping Review of the Animal Literature
Claire Fong-McMaster, Sandra Konji, Amanda Nitschke, Anne TM Konkle
International Journal of Environmental Research and Public Health.2020; 17(3): 926. CrossRef - Maternal exposure to arsenic and mercury and associated risk of adverse birth outcomes in small-scale gold mining communities in Northern Tanzania
Elias C. Nyanza, Deborah Dewey, Mange Manyama, Jonathan W. Martin, Jennifer Hatfield, Francois P. Bernier
Environment International.2020; 137: 105450. CrossRef - A combined spectroscopic and ab initio study of the transmetalation of a polyphenol as a potential purification strategy for food additives
Tuhin Kumar Maji, Damayanti Bagchi, Nivedita Pan, Ali Sayqal, Moataz Morad, Saleh A. Ahmed, Debjani Karmakar, Samir Kumar Pal
RSC Advances.2020; 10(10): 5636. CrossRef - Hematological parameters and hair mercury levels in adolescents from the Colombian Caribbean
Alejandra Manjarres-Suarez, Jesus Olivero-Verbel
Environmental Science and Pollution Research.2020; 27(12): 14216. CrossRef - A comparison of individual-level vs. hypothetically pooled mercury biomonitoring data from the Maternal Organics Monitoring Study (MOMS), Alaska, 1999-2012
Emily Mosites, Ernesto Rodriguez, Samuel P. Caudill, Thomas W. Hennessy, James Berner
International Journal of Circumpolar Health.2020;[Epub] CrossRef - Reference values for heavy metals in the urine and blood of Saudi women derived from two human biomonitoring studies
Iman Al-Saleh
International Journal of Hygiene and Environmental Health.2020; 225: 113473. CrossRef - Ecosystem-Scale Modeling and Field Observations of Sulfate and Methylmercury Distributions in the Florida Everglades: Responses to Reductions in Sulfate Loading
William H. Orem, Carl Fitz, David P. Krabbenhoft, Brett A. Poulin, Matthew S. Varonka, George R. Aiken
Aquatic Geochemistry.2020; 26(3): 191. CrossRef - From classic methodologies to application of nanomaterials for soil remediation: an integrated view of methods for decontamination of toxic metal(oid)s
Lilian Rodrigues Rosa Souza, Luiza Carolina Pomarolli, Márcia Andreia Mesquita Silva da Veiga
Environmental Science and Pollution Research.2020; 27(10): 10205. CrossRef - Effects of microplastics and mercury on manila clam Ruditapes philippinarum: Feeding rate, immunomodulation, histopathology and oxidative stress
Ercan Sıkdokur, Murat Belivermiş, Narin Sezer, Murat Pekmez, Ömür Karabulut Bulan, Önder Kılıç
Environmental Pollution.2020; 262: 114247. CrossRef - Thermal stress accelerates mercury chloride toxicity in Oreochromis niloticus via up-regulation of mercury bioaccumulation and HSP70 mRNA expression
Rania Waheed, Amel M. El Asely, Hatem Bakery, Ragab El-Shawarby, Mohamed Abuo-Salem, Nabila Abdel-Aleem, Farag Malhat, Asmaa Khafaga, Ahmed Abdeen
Science of The Total Environment.2020; 718: 137326. CrossRef - Metal oxide QD based ultrasensitive microsphere fluorescent sensor for copper, chromium and iron ions in water
Md. Motiar R. Khan, Tapas Mitra, Dibakar Sahoo
RSC Advances.2020; 10(16): 9512. CrossRef - Association between School Performance and Anemia in Adolescents in Mexico
Alejandro Mosiño, Karen P. Villagómez-Estrada, Alberto Prieto-Patrón
International Journal of Environmental Research and Public Health.2020; 17(5): 1466. CrossRef - Ultrasensitive determination of mercury ions using a glassy carbon electrode modified with nanocomposites consisting of conductive polymer and amino-functionalized graphene quantum dots
Bowen Tian, Yanxia Kou, Xiangmei Jiang, Jiajia Lu, Yuanyuan Xue, Meijuan Wang, Liang Tan
Microchimica Acta.2020;[Epub] CrossRef - The mercury level in hair and breast milk of lactating mothers in Iran: a systematic review and meta-analysis
Norouz Mahmoudi, Ahmad Jonidi Jafari, Yousef Moradi, Ali Esrafili
Journal of Environmental Health Science and Engineering.2020; 18(1): 355. CrossRef - Subchronic oral mercury caused intestinal injury and changed gut microbiota in mice
Yulan Zhao, Changming Zhou, Cong Wu, Xiaoquan Guo, Guoliang Hu, Qingpeng Wu, Zheng Xu, Guyue Li, Huabin Cao, Lin Li, Vincent Latigo, Pei Liu, Sufang Cheng, Ping Liu
Science of The Total Environment.2020; 721: 137639. CrossRef - A phenobarbital containing polymer/ silica coated quantum dot composite for the selective recognition of mercury species in fish samples using a room temperature phosphorescence quenching assay
Kamal K. Jinadasa, Elena Peña-Vázquez, Pilar Bermejo-Barrera, Antonio Moreda-Piñeiro
Talanta.2020; 216: 120959. CrossRef - Simulation of atmospheric mercury dispersion and deposition in Tehran city
Mohammadamin Vahidi Ghazvini, Khosro Ashrafi, Majid Shafiepour Motlagh, Alireza Pardakhti, Sarmad Ghader, Thomas M. Holsen
Air Quality, Atmosphere & Health.2020; 13(5): 529. CrossRef - Toxic Metal Implications on Agricultural Soils, Plants, Animals, Aquatic life and Human Health
Uchenna Okereafor, Mamookho Makhatha, Lukhanyo Mekuto, Nkemdinma Uche-Okereafor, Tendani Sebola, Vuyo Mavumengwana
International Journal of Environmental Research and Public Health.2020; 17(7): 2204. CrossRef - Impacts of Coal Use on Health
Michael Hendryx, Keith J. Zullig, Juhua Luo
Annual Review of Public Health.2020; 41(1): 397. CrossRef - Determination of (Bio)-available mercury in soils: A review
Jen-How Huang, Waleed H. Shetaya, Stefan Osterwalder
Environmental Pollution.2020; 263: 114323. CrossRef - Biosorption of Water Pollutants by Fungal Pellets
Adriana Legorreta-Castañeda, Carlos Lucho-Constantino, Rosa Beltrán-Hernández, Claudia Coronel-Olivares, Gabriela Vázquez-Rodríguez
Water.2020; 12(4): 1155. CrossRef - Metals, autoimmunity, and neuroendocrinology: Is there a connection?
Geir Bjørklund, Maryam Dadar, Salvatore Chirumbolo, Jan Aaseth, Massimiliano Peana
Environmental Research.2020; 187: 109541. CrossRef - Keratinous biomarker of mercury exposure associated with amyotrophic lateral sclerosis risk in a nationwide U.S. study
Angeline S. Andrew, Katie M. O’Brien, Brian P. Jackson, Dale P. Sandler, Wendy E. Kaye, Laurie Wagner, Elijah W. Stommel, D. Kevin Horton, Paul Mehta, Clarice R. Weinberg
Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration.2020; 21(5-6): 420. CrossRef - Methylmercury in Predatory and Non-predatory Fish Species Marketed in the Amazon Triple Frontier
Stephani Ferreira da Silva, João Paulo Góes Pereira, Diomar Cavalcante Oliveira, Marcelo de Oliveira Lima
Bulletin of Environmental Contamination and Toxicology.2020; 104(6): 733. CrossRef - Repression of mercury accumulation and adverse effects of methylmercury exposure is mediated by cystathionine γ-lyase to produce reactive sulfur species in mouse brain
Masahiro Akiyama, Takamitsu Unoki, Eiko Yoshida, Yunjie Ding, Hiroto Yamakawa, Yasuhiro Shinkai, Isao Ishii, Yoshito Kumagai
Toxicology Letters.2020; 330: 128. CrossRef - Fluorescent, colourimetric, and ratiometric probes based on diverse fluorophore motifs for mercuric(II) ion (Hg2+) sensing: highlights from 2011 to 2019
Stephen Opeyemi Aderinto
Chemical Papers.2020; 74(10): 3195. CrossRef - A pilot study to evaluate the levels of aqueous humor trace elements in open-angle glaucoma
Beatrice Bocca, Giovanni Forte, Andrea Pisano, Cristiano Farace, Ermete Giancipoli, Antonio Pinna, Stefano Dore, Roberto Madeddu
Journal of Trace Elements in Medicine and Biology.2020; 61: 126560. CrossRef - Natural radionuclides, heavy metals and health risk assessment in surface water of Nkalagu river dam with statistical analysis
Fredrick Oghenebrorie Ugbede, Bruno Chudy Aduo, Onyinyechi Nnenna Ogbonna, Ogechi Chinelo Ekoh
Scientific African.2020; 8: e00439. CrossRef - Development of aminoethylpyridine based N,N,N,O-donor fluorescent probes for the detection of Fe3+ and Hg2+ in aqueous media
Durgesh Singh, Neha Thakur, Krishna K. Raj, Rampal Pandey
Journal of Physics: Conference Series.2020; 1504: 012001. CrossRef - Evaluation of possible impact on human health of atmospheric mercury emanations from the Popocatépetl volcano
B. Schiavo, O. Morton-Bermea, E. Salgado-Martinez, E. Hernández-Álvarez
Environmental Geochemistry and Health.2020; 42(11): 3717. CrossRef -
Bioanode-Assisted Removal of Hg
2+
at the Cathode of Microbial Fuel Cells
Rohit Kumar, Sukrampal Yadav, Sunil A. Patil
Journal of Hazardous, Toxic, and Radioactive Waste.2020;[Epub] CrossRef - Total mercury in wild felids occurring in protected areas in the central Brazilian Amazon
Marcelly Castello Branco LOPES, Gabriel Oliveira de CARVALHO, Robson Roney BERNARDO, Joana MACEDO, Adan Santos LINO, Emiliano Esterci RAMALHO, Daniele KASPER, Rodrigo Ornellas MEIRE, João Paulo Machado TORRES, Olaf MALM
Acta Amazonica.2020; 50(2): 142. CrossRef - Mercury speciation based on mercury-stimulated peroxidase mimetic activity of gold nanoparticles
Jian-Yu Yang, Xin-Di Jia, Xiao-Yan Wang, Ming-Li Chen, Ting Yang, Jian-Hua Wang
The Analyst.2020; 145(15): 5200. CrossRef - Influence of Prenatal Exposure to Mercury, Perceived Stress, and Depression on Birth Outcomes in Suriname: Results from the MeKiTamara Study
Anisma R. Gokoel, Wilco C. W. R. Zijlmans, Hannah H. Covert, Firoz Abdoel Wahid, Arti Shankar, M. Sigrid MacDonald-Ottevanger, Ashna D. Hindori-Mohangoo, Jeffrey K. Wickliffe, Maureen Y. Lichtveld, Emily W. Harville
International Journal of Environmental Research and Public Health.2020; 17(12): 4444. CrossRef - Determination of the Total Content of Arsenic, Antimony, Selenium and Mercury in Chinese Herbal Food by Chemical Vapor Generation-Four-Channel Non-dispersive Atomic Fluorescence Spectrometry
Xu Deng, Risheng Li, Shupei Deng
Journal of Fluorescence.2020; 30(4): 949. CrossRef - Trophic Magnification of Legacy (PCB, DDT and Hg) and Emerging Pollutants (PFAS) in the Fish Community of a Small Protected Southern Alpine Lake (Lake Mergozzo, Northern Italy)
Michela Mazzoni, Claudia Ferrario, Roberta Bettinetti, Roberta Piscia, Davide Cicala, Pietro Volta, Katrine Borgå, Sara Valsecchi, Stefano Polesello
Water.2020; 12(6): 1591. CrossRef - Do Two Wrongs Make a Right? Persistent Uncertainties Regarding Environmental Selenium–Mercury Interactions
Jacqueline R. Gerson, David M. Walters, Collin A. Eagles-Smith, Emily S. Bernhardt, Jessica E. Brandt
Environmental Science & Technology.2020; 54(15): 9228. CrossRef - A Highly Selective Fluorescent Probe for Hg2+ Based on a 1,8-Naphthalimide Derivative
Meiju Tian, Chunyan Wang, Qiujuan Ma, Yu Bai, Jingguo Sun, Chunfeng Ding
ACS Omega.2020; 5(29): 18176. CrossRef - Neuropsychological effects of long-term occupational exposure to mercury among chloralkali workers
Majid Bagheri Hosseinabadi, Narges Khanjani, Mostafa Dehghani Mobarake, Hamid Shirkhanloo
Work.2020; 66(3): 491. CrossRef - An overview of nanoscale materials on the removal of wastewater contaminants
Ramendra Soni, Arun Kumar Pal, Pooja Tripathi, Jonathan A. Lal, Kavindra Kesari, Vijay Tripathi
Applied Water Science.2020;[Epub] CrossRef - Mercury removal by porous sulfur copolymers: Adsorption isotherm and kinetics studies
Vijay S. Wadi, Hemant Mittal, E. Fosso-Kankeu, Kishore K. Jena, Saeed M. Alhassan
Colloids and Surfaces A: Physicochemical and Engineering Aspects.2020; 606: 125333. CrossRef - Mercury Exposure and Health Problems of the Students Using Skin-Lightening Cosmetic Products in Makassar, South Sulawesi, Indonesia
Hasriwiani Habo Abbas, Masayuki Sakakibara, Koichiro Sera, Nurgahayu, Ella Andayanie
Cosmetics.2020; 7(3): 58. CrossRef - Novel Insights into Mercury Effects on Hemoglobin and Membrane Proteins in Human Erythrocytes
Marina Piscopo, Rosaria Notariale, Fabiana Tortora, Gennaro Lettieri, Giancarlo Palumbo, Caterina Manna
Molecules.2020; 25(14): 3278. CrossRef - Biotransformation fate and sustainable mitigation of a potentially toxic element of mercury from environmental matrices
Pengfei Duan, Suliman Khan, Nisar Ali, Muhammad Adnan Shereen, Rabeea Siddique, Barkat Ali, Hafiz M.N. Iqbal, Ghulam Nabi, Wasim Sajjad, Muhammad Bilal
Arabian Journal of Chemistry.2020; 13(9): 6949. CrossRef - A study on the potential reprotoxic effects of thimerosal in male albino rats
Muhammad Umar Ijaz, Moazama Batool, Asma Ashraf, Muhammad Hussnain Siddique, Sara Zafar, Saima Muzammil, Fatima Ayaz, Abdul Samad, Khalid Al-Ghanim, Shahid Mahboob
Saudi Journal of Biological Sciences.2020; 27(10): 2798. CrossRef - The Stakeholder’s Position Map Related to the Mercury Pollution Reduction Program in Bombana Area, Southeast Sulawesi, Indonesia
Basri, Masayuki Sakakibara
IOP Conference Series: Earth and Environmental Science.2020; 536(1): 012008. CrossRef - Regulation of Single-Channel Conductance of Voltage-Dependent Anion Channel by Mercuric Chloride in a Planar Lipid Bilayer
Chetan Malik, Subhendu Ghosh
The Journal of Membrane Biology.2020; 253(4): 357. CrossRef - Morphological abnormalities in fish parasites: a potential tool for biomonitoring natural contaminants?
Tímea Brázová, Martina Orosová, Peter Šalamún, Vladimíra Hanzelová
Parasitology Research.2020; 119(10): 3297. CrossRef - Robust Structurally Colored Coatings Composed of Colloidal Arrays Prepared by the Cathodic Electrophoretic Deposition Method with Metal Cation Additives
Kiyofumi Katagiri, Kensuke Uemura, Ryo Uesugi, Naoki Tarutani, Kei Inumaru, Tetsuo Uchikoshi, Takahiro Seki, Yukikazu Takeoka
ACS Applied Materials & Interfaces.2020; 12(36): 40768. CrossRef - Environmental influence on neurodevelopmental disorders: Potential association of heavy metal exposure and autism
Omamuyovwi M. Ijomone, Nzube F. Olung, Grace T. Akingbade, Comfort O.A. Okoh, Michael Aschner
Journal of Trace Elements in Medicine and Biology.2020; 62: 126638. CrossRef - Synthesis, structural characterization and evaluation of the chelating potential in C. elegans involving complexes of mercury (II) with Schiff bases derived from amino acids
Leandro de O. Amaral, Viner Sousa Lima, Sérgio Macêdo Soares, Julia Bornhorst, Sebastião S. Lemos, Claudia Cristina Gatto, Robert A. Burrow, Priscila Gubert
Journal of Organometallic Chemistry.2020; 926: 121500. CrossRef - Relationship between mercury and selenium concentrations in tissues from stranded odontocetes in the northern Gulf of Mexico
Meaghan A. McCormack, Brian P. Jackson, Jessica Dutton
Science of The Total Environment.2020; 749: 141350. CrossRef - Colorimetric and fluorescent multi-ion recognition by Anthracene appended di-Schiff base chemosensor
Navneet Kaur, Baljeet Kaur
Inorganic Chemistry Communications.2020; 121: 108239. CrossRef - Mercury level in biological samples of dentists in Iran: a systematic review and meta-analysis
Ahmad Jonidi Jafari, Ali Esrafili, Yousef Moradi, Norouz Mahmoudi
Journal of Environmental Health Science and Engineering.2020; 18(2): 1655. CrossRef - Spatial and Temporal Trends of Gaseous Elemental Mercury over a Highly Impacted Coastal Environment (Northern Adriatic, Italy)
Nicolò Barago, Federico Floreani, Alessandro Acquavita, José María Esbrí, Stefano Covelli, Pablo Higueras
Atmosphere.2020; 11(9): 935. CrossRef - Inorganic ions in the skin: Allies or enemies?
Małgorzata Tarnowska, Stéphanie Briançon, Jacqueline Resende de Azevedo, Yves Chevalier, Marie-Alexandrine Bolzinger
International Journal of Pharmaceutics.2020; 591: 119991. CrossRef - Ethanol leaf extract of Ruspolia hypocrateriformis abrogated hepatic redox imbalance and oxidative damage induced by heavy metal toxicity in rats
J.N. Awoke, O.U. Orji, P.M. Aja, N.N. Ezeani, C. Aloke, O.D. Obasi
Arabian Journal of Chemistry.2020; 13(11): 8133. CrossRef - Thermodynamics, a suitable reporter in the design of mercury (II) ion selective electrodes
Nawal Al Hakawati, Salman S. Alharthi, Angela F. Danil de Namor
Arabian Journal of Chemistry.2020; 13(12): 8671. CrossRef - Elevated accumulation of the toxic metal mercury in the Critically Endangered oceanic whitetip shark Carcharhinus longimanus from the northwestern Atlantic Ocean
J Gelsleichter, G Sparkman, LA Howey, EJ Brooks, ON Shipley
Endangered Species Research.2020; 43: 267. CrossRef - Perfluoroalkyl substances (PFASs) and mercury in never-pregnant women of fertile age: association with fish consumption and unfavorable lipid profile
Anne-Lise Bjorke-Monsen, Kristin Varsi, Maria Averina, Jan Brox, Sandra Huber
BMJ Nutrition, Prevention & Health.2020; 3(2): 277. CrossRef - Versatile Colorimetric Chemosensor Based on Bis(rhodamine) B Hydrazone for Rapid Detection of Multi-Analytes (Fe3+, Bi3+, Cu2+, and Hg2+) in Aqueous Media
Tamer El Malah, Hany F. Nour
Australian Journal of Chemistry.2020; 73(11): 1112. CrossRef - Urinary Mercury Levels and Predictors of Exposure among a Group of Italian Children
Maria Luisa Astolfi, Matteo Vitali, Elisabetta Marconi, Stefano Martellucci, Vincenzo Mattei, Silvia Canepari, Carmela Protano
International Journal of Environmental Research and Public Health.2020; 17(24): 9225. CrossRef - Adaptive mechanisms induced by sparingly soluble mercury sulfide (HgS) in zebrafish: behavioural and proteomics analysis
Snehasis Biswas, Jayesh Bellare
Chemosphere.2020; : 129438. CrossRef - Mercury in aquatic fauna contamination: A systematic review on its dynamics and potential health risks
Paloma de Almeida Rodrigues, Rafaela Gomes Ferrari, Luciano Neves dos Santos, Carlos Adam Conte Junior
Journal of Environmental Sciences.2019; 84: 205. CrossRef - Cysteamine-modified diamond nanoparticles applied in cellular imaging and Hg2+ ions detection
Muthaiah Shellaiah, Turibius Simon, Parthiban Venkatesan, Kien Wen Sun, Fu-Hsiang Ko, Shu-Pao Wu
Applied Surface Science.2019; 465: 340. CrossRef - Calcium Channels, Rho-Kinase, Protein Kinase-C, and Phospholipase-C Pathways Mediate Mercury Chloride-Induced Myometrial Contractions in Rats
Swati Koli, Atul Prakash, Soumen Choudhury, Rajesh Mandil, Satish K. Garg
Biological Trace Element Research.2019; 187(2): 418. CrossRef - Mercury Involvement in Neuronal Damage and in Neurodegenerative Diseases
Veronica Lanza Cariccio, Annalisa Samà, Placido Bramanti, Emanuela Mazzon
Biological Trace Element Research.2019; 187(2): 341. CrossRef - Ambient mercury source identification at a New York State urban site: Rochester, NY
Hao Zhou, Philip K. Hopke, Chuanlong Zhou, Thomas M. Holsen
Science of The Total Environment.2019; 650: 1327. CrossRef - Electrogenerated chemiluminescence biosensor for detection of mercury (II) ion via target-triggered manipulation of DNA three-way junctions
Fen Ma, Yu Chen, Yinchang Zhu, Jiawei Liu
Talanta.2019; 194: 114. CrossRef - Effects of temperature and SO3 on re-emission of mercury from activated carbon under flue gas conditions
Michael Royko, Benjamin Galloway, Noah D. Meeks, Bihter Padak
Journal of Environmental Sciences.2019; 79: 67. CrossRef - Prenatal and Early Postnatal Exposure to Total Mercury and Methylmercury from Low Maternal Fish Consumption
Monika Ursinyova, Vlasta Masanova, Iveta Uhnakova, Lubica Palkovicova Murinova, Henrieta Patayova, Katarina Rausova, Tomas Trnovec, Jan Stencl, Martin Gajdos
Biological Trace Element Research.2019; 191(1): 16. CrossRef - Mercury pollution in modern times and its socio-medical consequences
Lygia Therese Budnik, Ludwine Casteleyn
Science of The Total Environment.2019; 654: 720. CrossRef - Chronic mercury exposure and blood pressure in children and adolescents: a systematic review
Gema Gallego-Viñas, Ferran Ballester, Sabrina Llop
Environmental Science and Pollution Research.2019; 26(3): 2238. CrossRef - Photocatalytic reduction and scavenging of Hg(II) over templated-dewetted Au on TiO2 nanotubes
Davide Spanu, Alessandro Bestetti, Helga Hildebrand, Patrik Schmuki, Marco Altomare, Sandro Recchia
Photochemical & Photobiological Sciences.2019; 18(5): 1046. CrossRef - Neurochemical dysfunction in motor cortex and hippocampus impairs the behavioral performance of rats chronically exposed to inorganic mercury
Francisco Bruno Teixeira, Luana Ketlen Reis Leão, Leonardo Oliveira Bittencourt, Walessa Alana Bragança Aragão, Priscila Cunha Nascimento, Diandra Araújo Luz, Danielle Valente Braga, Márcia Cristina Freitas da Silva, Karen Renata Matos Oliveira, Anderson
Journal of Trace Elements in Medicine and Biology.2019; 52: 143. CrossRef - Silver Nanoparticle-Loaded Activated Carbon as an Adsorbent for the Removal of Mercury from Arabian Gas-Condensate
Salawu Omobayo Adio, Azeem Rana, Basheer Chanabsha, Abdulmalik Adil Khalid BoAli, Mohammad Essa, Abdulaziz Alsaadi
Arabian Journal for Science and Engineering.2019; 44(7): 6285. CrossRef - Application of micellar catalysis in ultrasensitive quantification of drotaverine hydrochloride
Kshiti Singh, Ankita Sinha, Rajeev Jain
Ionics.2019; 25(7): 3419. CrossRef - Mercury chloride toxicity in human erythrocytes: enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system
Shahbaz Ahmad, Riaz Mahmood
Environmental Science and Pollution Research.2019; 26(6): 5645. CrossRef - Seawater intrusion and resuspension of surface sediment control mercury (Hg) distribution and its bioavailability in water column of a monsoonal estuarine system
Parthasarathi Chakraborty, Saranya Jayachandran, Jyothi Lekshmy, Prasad Padalkar, Lamjahao Sitlhou, Kartheek Chennuri, Suhas Shetye, Areef Sardar, Rakhee Khandeparker
Science of The Total Environment.2019; 660: 1441. CrossRef - A novel 1,8-naphthalimide as highly selective naked-eye and ratiometric fluorescent sensor for detection of Hg2+ ions
Muzey Bahta, Naseem Ahmed
Journal of Photochemistry and Photobiology A: Chemistry.2019; 373: 154. CrossRef - Bioconcentration and translocation of Cd and Hg in a tomato (Solanum lycopersicum) from cultivated soils in southeastern Brazil
Clara A. I. Lima, Inacio A. Pestana, Lucas S. Azevedo, Daniel P. Ribeiro, Marcelo G. Almeida, Claudia L. Prins, Claudio R. Marciano, Cristina M. M. Souza
Environmental Monitoring and Assessment.2019;[Epub] CrossRef - Human exposure to mercury and its hematological effects: a systematic review
Angélica dos Santos Vianna, Elisabete Pedra de Matos, Iracina Maura de Jesus, Carmen Ildes Rodrigues Fróes Asmus, Volney de Magalhães Câmara
Cadernos de Saúde Pública.2019;[Epub] CrossRef - Environmental impacts of the life cycle of alluvial gold mining in the Peruvian Amazon rainforest
Ramzy Kahhat, Eduardo Parodi, Gustavo Larrea-Gallegos, Carlos Mesta, Ian Vázquez-Rowe
Science of The Total Environment.2019; 662: 940. CrossRef - Mercury-induced inflammation and autoimmunity
K. Michael Pollard, David M. Cauvi, Christopher B. Toomey, Per Hultman, Dwight H. Kono
Biochimica et Biophysica Acta (BBA) - General Subjects.2019; 1863(12): 129299. CrossRef - An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects
Brij Mohan Sharma, Ondřej Sáňka, Jiří Kalina, Martin Scheringer
Environment International.2019; 125: 300. CrossRef - Docosahexaenoic acid enhances methylmercury-induced endoplasmic reticulum stress and cell death and eicosapentaenoic acid potentially attenuates these effects in mouse embryonic fibroblasts
Yasukazu Takanezawa, Ryosuke Nakamura, Miho Hamaguchi, Kanae Yamamoto, Yuka Sone, Shimpei Uraguchi, Masako Kiyono
Toxicology Letters.2019; 306: 35. CrossRef - A multidimensional concept for mercury neuronal and sensory toxicity in fish - From toxicokinetics and biochemistry to morphometry and behavior
Patrícia Pereira, Malgorzata Korbas, Vitória Pereira, Tiziana Cappello, Maria Maisano, João Canário, Armando Almeida, Mário Pacheco
Biochimica et Biophysica Acta (BBA) - General Subjects.2019; 1863(12): 129298. CrossRef - Assessing effects of germline exposure to environmental toxicants by high-throughput screening in C. elegans
Nara Shin, Luciann Cuenca, Rajendiran Karthikraj, Kurunthachalam Kannan, Monica P. Colaiácovo, Gregory P. Copenhaver
PLOS Genetics.2019; 15(2): e1007975. CrossRef - Invisible and ignored? Local perspectives on mercury in Congolese gold mining
Bossissi Nkuba, Lieven Bervoets, Sara Geenen
Journal of Cleaner Production.2019; 221: 795. CrossRef - Recent development of carbon quantum dots regarding their optical properties, photoluminescence mechanism, and core structure
Keenan J. Mintz, Yiqun Zhou, Roger M. Leblanc
Nanoscale.2019; 11(11): 4634. CrossRef - Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review
David O'Connor, Deyi Hou, Yong Sik Ok, Jan Mulder, Lei Duan, Qingru Wu, Shuxiao Wang, Filip M.G. Tack, Jörg Rinklebe
Environment International.2019; 126: 747. CrossRef - Total mercury, chromium, nickel and other trace chemical element contents in soils at an old cinnabar mine site (Merník, Slovakia): anthropogenic versus natural sources of soil contamination
Tatsiana Kulikova, Edgar Hiller, Ľubomír Jurkovič, Lenka Filová, Peter Šottník, Petr Lacina
Environmental Monitoring and Assessment.2019;[Epub] CrossRef - Exposure routes and health effects of heavy metals on children
Muwaffak Al osman, Fei Yang, Isaac Yaw Massey
BioMetals.2019; 32(4): 563. CrossRef - Reflective Fiber Surface Plasmon Resonance Sensor for High-Sensitive Mercury Ion Detection
Zhenlin Chen, Kunlin Han, Ya-Nan Zhang
Applied Sciences.2019; 9(7): 1480. CrossRef - Multiple Lines of Evidences Reveal Mechanisms Underpinning Mercury Resistance and Volatilization by Stenotrophomonas sp. MA5 Isolated from the Savannah River Site (SRS), USA
Meenakshi Agarwal, Rajesh Singh Rathore, Charles Jagoe, Ashvini Chauhan
Cells.2019; 8(4): 309. CrossRef - Protective Effects ofPlathymenia reticulataandConnarus favosusAqueous Extracts against Cadmium- and Mercury-Induced Toxicities
Kewin Gombeau, Ricardo Bezerra de Oliveira, Sandra Layse Ferreira Sarrazin, Rosa Helena Veras Mourão, Jean-Paul Bourdineaud
Toxicological Research.2019; 35(1): 25. CrossRef - Mercurio, metilmercurio y otros metales pesados en peces de Colombia: riesgo por ingesta
Shirly Paola Vargas Licona, José Luis Marrugo Negrete
Acta Biológica Colombiana.2019; 24(2): 232. CrossRef - Exposição ocupacional ao mercúrio em cooperativas de triagem de materiais recicláveis da região metropolitana de São Paulo, SP, Brasil
Nelson Gouveia, Marcia Liane Buzzo, Maria Gricia de Lourdes Grossi, Gisele Ferreira de Souza, Elizabeti Yuriko Muto
Ciência & Saúde Coletiva.2019; 24(4): 1517. CrossRef - Instant Mercury Ion Detection in Industrial Waste Water with a Microchip Using Extended Gate Field-Effect Transistors and a Portable Device
Revathi Sukesan, Yi-Ting Chen, Suman Shahim, Shin-Li Wang, Indu Sarangadharan, Yu-Lin Wang
Sensors.2019; 19(9): 2209. CrossRef - Preparation and characterization of Ca(II) cross-linking modified pectin microspheres for Pb(II) adsorption
Fen Li, Zhao Xu, Xiaoyan Wen, Xiaoyong Li, Yanhong Bai, Jianjun Li
Water Science and Technology.2019; 79(8): 1484. CrossRef - Minireview: Plausible Applications of Chemical Sensors for the Detection of Toxic Metal Ions
Mikolaj Wituszyñski, Shiva Prasad Kollur
Analytical Chemistry Letters.2019; 9(2): 113. CrossRef - Bioremediation treatment process through mercury-resistant bacteria isolated from Mithi river
Bhupendra Pushkar, Pooja Sevak, Akansha Singh
Applied Water Science.2019;[Epub] CrossRef - Mercury Contamination from Dental Amalgam
Anita Vazquez Tibau, Blanche D. Grube
Journal of Health and Pollution.2019;[Epub] CrossRef - Protective effect of high zinc levels on preterm birth induced by mercury exposure during pregnancy: A birth cohort study in China
Haiyun Xiang, Yiran Tao, Baoli Zhang, Chunmei Liang, Zhijuan Li, Lanlan Feng, Juan Qi, Wan Pan, Juan Tong, Shuangqin Yan, Fangbiao Tao
Journal of Trace Elements in Medicine and Biology.2019; 55: 71. CrossRef - Nanocatalyst/Nanoplasmon‐Enabled Detection of Organic Mercury: A One‐Minute Visual Test
Paolo Donati, Mauro Moglianetti, Marina Veronesi, Mirko Prato, Giuseppina Tatulli, Tiziano Bandiera, Pier Paolo Pompa
Angewandte Chemie International Edition.2019; 58(30): 10285. CrossRef - Using H2S plasma to modify activated carbon for elemental mercury removal
Mengting Shi, Guangqian Luo, Yang Xu, Renjie Zou, Hailu Zhu, Jingyuan Hu, Xian Li, Hong Yao
Fuel.2019; 254: 115549. CrossRef - Elemental Analysis of Aging Human Pituitary Glands Implicates Mercury as a Contributor to the Somatopause
Roger Pamphlett, Stephen Kum Jew, Philip A. Doble, David P. Bishop
Frontiers in Endocrinology.2019;[Epub] CrossRef - Effect of combination of Tribulus terrestris, Boerhavia diffusa and Terminalia chebula reverses mercuric chloride-induced nephrotoxicity and renal accumulation of mercury in rat
Harlokesh Narayan Yadav, Uma Shankar Sharma, Surender Singh, Yogendra Kumar Gupta
Oriental Pharmacy and Experimental Medicine.2019; 19(4): 497. CrossRef - A novel hybrid long period fiber grating-diffusive gradient in thin films sensor system for the detection of mercury (II) ions in water
Shin-Yinn Tan, Sheng-Chyan Lee, Hideki Kuramitz, Faidz Abd-Rahman
Optik.2019; 194: 163040. CrossRef - Water-soluble mercury induced by organic amendments affected microbial community assemblage in mercury-polluted paddy soil
Hualing Hu, Meng Li, Guoxi Wang, Marios Drosos, Zhen Li, Zhengyi Hu, Beidou Xi
Chemosphere.2019; 236: 124405. CrossRef - Micro- and Mesoporous Carbons Derived from KOH Activations of Polycyanurates with High Adsorptions for CO2 and Iodine
Shih-Ting Wang, Jin-Long Hong
ACS Omega.2019; 4(7): 12018. CrossRef - Methylmercury exposure induces ROS/Akt inactivation-triggered endoplasmic reticulum stress-regulated neuronal cell apoptosis
Yao-Pang Chung, Cheng-Chieh Yen, Feng-Cheng Tang, Kuan-I Lee, Shing-Hwa Liu, Chin-Ching Wu, Shang-Shu Hsieh, Chin-Chuan Su, Chun-Ying Kuo, Ya-Wen Chen
Toxicology.2019; 425: 152245. CrossRef - Seasonal variation of mercury in commercial fishes of the Amazon Triple Frontier, Western Amazon Basin
Stephani Ferreira da Silva, Diomar Cavalcante Oliveira, João Paulo Góes Pereira, Simone Pinto Castro, Brenda Natasha Souza Costa, Marcelo de Oliveira Lima
Ecological Indicators.2019; 106: 105549. CrossRef - Simultaneous determination of noble metals, Sb and Hg by magnetic solid phase extraction on line ICP OES based on a new functionalized magnetic graphene oxide
J.C García-Mesa, P. Montoro Leal, M.M López Guerrero, E.I Vereda Alonso
Microchemical Journal.2019; 150: 104141. CrossRef - Levels of mercury in Nile tilapia (Oreochromis niloticus), water, and sediment in the Migori gold mining belt, Kenya, and the potential ramifications to human health
Samwel Kola, Laetitia Wakonyu Kanja, James Mucunu Mbaria, Joyce Gichiku Maina, Mitchel Otieno Okumu
F1000Research.2019; 8: 1244. CrossRef - Reversible alopecia associated with high blood mercury levels and early menopause: a report of two cases
Jane B. Peters, Michelle P. Warren
Menopause.2019; 26(8): 915. CrossRef - Detection of Al3+ and Hg2+ ions with anthracene appended Schiff base and its reduced analogue
Baljeet Kaur, Navneet Kaur
Journal of Coordination Chemistry.2019; 72(13): 2189. CrossRef - Mercury species in the nests and bodies of soil-feeding termites, Silvestritermes spp. (Termitidae, Syntermitinae), in French Guiana
Michel Diouf, David Sillam-Dussès, Vanessa Alphonse, Sophie Frechault, Edouard Miambi, Philippe Mora
Environmental Pollution.2019; 254: 113064. CrossRef - Critical review of mercury contamination in Sri Lankan fish and aquatic products
B.K.K.K. Jinadasa, Scott W. Fowler
Marine Pollution Bulletin.2019; 149: 110526. CrossRef - Colorimetric and Fluorescence-Based Detection of Mercuric Ion Using a Benzothiazolinic Spiropyran
Ajeet Kumar, Arvind Kumar, Priya Ranjan Sahoo, Satish Kumar
Chemosensors.2019; 7(3): 35. CrossRef - Nanocatalyst/Nanoplasmon‐Enabled Detection of Organic Mercury: A One‐Minute Visual Test
Paolo Donati, Mauro Moglianetti, Marina Veronesi, Mirko Prato, Giuseppina Tatulli, Tiziano Bandiera, Pier Paolo Pompa
Angewandte Chemie.2019; 131(30): 10391. CrossRef - Microbial mercury methylation in the cryosphere: Progress and prospects
Prakriti Sharma Ghimire, Lekhendra Tripathee, Qianggong Zhang, Junming Guo, Kirpa Ram, Jie Huang, Chhatra Mani Sharma, Shichang Kang
Science of The Total Environment.2019; 697: 134150. CrossRef - A Review on Coordination Properties of Thiol-Containing Chelating Agents Towards Mercury, Cadmium, and Lead
Geir Bjørklund, Guido Crisponi, Valeria Marina Nurchi, Rosita Cappai, Aleksandra Buha Djordjevic, Jan Aaseth
Molecules.2019; 24(18): 3247. CrossRef - Before the beginning: environmental exposures and reproductive and obstetrical outcomes
Thalia R. Segal, Linda C. Giudice
Fertility and Sterility.2019; 112(4): 613. CrossRef - Incorporation of ZnO/Bioactive Glass Nanoparticles into Alginate/Chitosan Composite Hydrogels for Wound Closure
Jiangying Zhu, Guohua Jiang, Gao Song, Tianqi Liu, Cong Cao, Yuhui Yang, Yajing Zhang, Wenjie Hong
ACS Applied Bio Materials.2019; 2(11): 5042. CrossRef - Mercury chloride exposure induces DNA damage, reduces fertility, and alters somatic and germline cells in Drosophila melanogaster ovaries
Luis Humberto Mojica-Vázquez, Diana Madrigal-Zarraga, Rocío García-Martínez, Muriel Boube, María Elena Calderón-Segura, Justine Oyallon
Environmental Science and Pollution Research.2019; 26(31): 32322. CrossRef - Health Risk Assessment of Heavy Metals and Lipid Quality Indexes in Freshwater Fish from Lakes of Warmia and Mazury Region, Poland
Joanna Łuczyńska, Beata Paszczyk
International Journal of Environmental Research and Public Health.2019; 16(19): 3780. CrossRef - Eco-potential of Aspergillus penicillioides (F12): bioremediation and antibacterial activity
Kishalay Paria, Susanta Kumar Chakraborty
SN Applied Sciences.2019;[Epub] CrossRef - Colorimetric sensing of Fe3+ and Hg2+ and photocatalytic activity of green synthesized silver nanoparticles from the leaf extract of Sonchus arvensis L.
Sandip Kumar Chandraker, Mithun Kumar Ghosh, Mishri Lal, Tanmay Kumar Ghorai, Ravindra Shukla
New Journal of Chemistry.2019; 43(46): 18175. CrossRef - Mercury Concentration in Technosols and Alder Tissue from a Plantation on a Combustion Waste Disposal Site
Bartłomiej Woś, Katarzyna Sroka, Agnieszka Józefowska, Marcin Pietrzykowski
Water, Air, & Soil Pollution.2019;[Epub] CrossRef - The first coordination polymers with an [O]2[N]P(S)-Hg segment: a combined experimental, theoretical and database study
Elham Torabi Farkhani, Mehrdad Pourayoubi, Mohammad Izadyar, Pavel V. Andreev, Ekaterina S. Shchegravina
Dalton Transactions.2019; 48(48): 17908. CrossRef - Ameliorating potency of Chenopodium album Linn. and vitamin C against mercuric chloride-induced oxidative stress in testes of Sprague Dawley rats
Sarwat Jahan, Tayyaba Azad, Amina Ayub, Asad Ullah, Tayyaba Afsar, Ali Almajwal, Suhail Razak
Environmental Health and Preventive Medicine.2019;[Epub] CrossRef - Emise rtuti, její antropogenní zdroje, environmentální a zdravotní rizika
Tomáš Ružovič, Karel Svoboda, MIchael Pohořelý
Paliva.2019; : 102. CrossRef - Emise rtuti, její antropogenní zdroje, environmentální a zdravotní rizika
Tomáš Ružovič
Paliva.2019; : 102. CrossRef - EFFECT AND OUTCOME OF HARMFUL TRADITIONAL PRACTICE OF ORAL ADMINISTRATION OF MERCURY IN NEWBORN AND INFANTS
Chidambaranathan Sivaprakasam, Ragavendran Jagannathan, Logesvar Palanisamy, Ramesh Samikannu
Journal of Evolution of Medical and Dental Sciences.2019; 8(2): 114. CrossRef - Mercury soil contents and associated ecological and health risks in kindergartens and functional areas of the city of Vanadzor (Armenia)
Lilit Sahakyan, Gevorg Tepanosyan, Gayane Melkonyan, Nairuhi Maghakyan, Armen Saghatelyan
GEOGRAPHY, ENVIRONMENT, SUSTAINABILITY.2019; 12(4): 252. CrossRef - A Rapid Assessment of the Impacts of Gold Mining on Women’s Health and Quality of Life in Ashanti Region, Ghana
Baraka Muvuka, Muriel J. Harris
Journal of Public Health Issues and Practices.2019;[Epub] CrossRef - Hazardous properties and toxicological update of mercury: From fish food to human health safety perspective
Charles Odilichukwu R. Okpala, Giacomo Sardo, Sergio Vitale, Gioacchino Bono, Augustine Arukwe
Critical Reviews in Food Science and Nutrition.2018; 58(12): 1986. CrossRef - Technical properties of biomass and solid recovered fuel (SRF) co-fired with coal: Impact on multi-dimensional resource recovery value
Eleni Iacovidou, John Hahladakis, Innes Deans, Costas Velis, Phil Purnell
Waste Management.2018; 73: 535. CrossRef - The Putative Role of Environmental Mercury in the Pathogenesis and Pathophysiology of Autism Spectrum Disorders and Subtypes
G. Morris, B. K. Puri, R. E. Frye, M. Maes
Molecular Neurobiology.2018; 55(6): 4834. CrossRef - Use of microwave irradiation for modification of mesoporous silica nanoparticles by thioglycolic acid for removal of cadmium and mercury
I.M.M. Kenawy, Y.G. Abou El-Reash, M.M. Hassanien, N.R. Alnagar, W.I. Mortada
Microporous and Mesoporous Materials.2018; 258: 217. CrossRef - Variation in fish mercury concentrations in streams of the Adirondack region, New York: A simplified screening approach using chemical metrics
Douglas A. Burns, Karen Riva-Murray
Ecological Indicators.2018; 84: 648. CrossRef - Carbon nanotubes magnetic hybrid nanocomposites for a rapid and selective preconcentration and clean-up of mercury species in water samples
Ana I. Corps Ricardo, Armando Sánchez-Cachero, María Jiménez-Moreno, Francisco J. Guzmán Bernardo, Rosa C. Rodríguez Martín-Doimeadios, Ángel Ríos
Talanta.2018; 179: 442. CrossRef - Mercury removal from MSW incineration flue gas by mineral-based sorbents
M. Rumayor, K. Svoboda, J. Švehla, M. Pohořelý, M. Šyc
Waste Management.2018; 73: 265. CrossRef - Recyclable Multifunctional Magnetic Mesoporous Silica Nanocomposite for Ratiometric Detection, Rapid Adsorption, and Efficient Removal of Hg(II)
Yanyan Wang, Meiyao Tang, He Shen, Guangbo Che, Yu Qiao, Bo Liu, Li Wang
ACS Sustainable Chemistry & Engineering.2018; 6(2): 1744. CrossRef - Chelator combination as therapeutic strategy in mercury and lead poisonings
Jan Aaseth, Olga P. Ajsuvakova, Anatoly V. Skalny, Margarita G. Skalnaya, Alexey A. Tinkov
Coordination Chemistry Reviews.2018; 358: 1. CrossRef - Trace elements in ALS patients and their relationships with clinical severity
Riccardo Oggiano, Giuliana Solinas, Giovanni Forte, Beatrice Bocca, Cristiano Farace, Andrea Pisano, Maria Alessandra Sotgiu, Simonetta Clemente, Michele Malaguarnera, Alessandro Giuseppe Fois, Pietro Pirina, Andrea Montella, Roberto Madeddu
Chemosphere.2018; 197: 457. CrossRef - The Content of Mercury in Herbal Dietary Supplements
Barbara Brodziak-Dopierała, Agnieszka Fischer, Wioletta Szczelina, Jerzy Stojko
Biological Trace Element Research.2018; 185(1): 236. CrossRef - Comparative neurotoxicity study of mercury-based inorganic compounds including Ayurvedic medicines Rasasindura and Kajjali in zebrafish model
Snehasis Biswas, Naay Balodia, Jayesh Bellare
Neurotoxicology and Teratology.2018; 66: 25. CrossRef - Quantitative estimation of mercury intake by toxicokinetic modelling based on total mercury levels in humans
K. Abass, A. Huusko, H.K. Knutsen, P. Nieminen, P. Myllynen, H.M. Meltzer, K. Vahakangas, A. Rautio
Environment International.2018; 114: 1. CrossRef - Oil extraction in the Amazon basin and exposure to metals in indigenous populations
Cristina O’Callaghan-Gordo, Juan A. Flores, Pilar Lizárraga, Tami Okamoto, Diana M. Papoulias, Federica Barclay, Martí Orta-Martínez, Manolis Kogevinas, John Astete
Environmental Research.2018; 162: 226. CrossRef - Historical atmospheric pollution trends in Southeast Asia inferred from lake sediment records
S. Engels, L.S.R.Z. Fong, Q. Chen, M.J. Leng, S. McGowan, M. Idris, N.L. Rose, M.S. Ruslan, D. Taylor, H. Yang
Environmental Pollution.2018; 235: 907. CrossRef - Metal Ion Effects on Aβ and Tau Aggregation
Anne Claire Kim, Sungsu Lim, Yun Kyung Kim
International Journal of Molecular Sciences.2018; 19(1): 128. CrossRef - Isoquinolinylpyrazoles and pyridylisoxazoles as luminescent materials with sensorial ability towards pollutant toxic metal ions. Experimental and computational studies
Cristián Cuerva, Nuno Morais, José A. Campo, Mercedes Cano, Carlos Lodeiro
Journal of Luminescence.2018; 198: 517. CrossRef - Detection of mercury (II) ions in water by polyelectrolyte–gold nanoparticles coated long period fiber grating sensor
Shin-Yinn Tan, Sheng-Chyan Lee, Takuya Okazaki, Hideki Kuramitz, Faidz Abd-Rahman
Optics Communications.2018; 419: 18. CrossRef - Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children
Jannike Øyen, Ingrid Kvestad, Lisa Kolden Midtbø, Ingvild Eide Graff, Mari Hysing, Kjell Morten Stormark, Maria Wik Markhus, Valborg Baste, Livar Frøyland, Berthold Koletzko, Hans Demmelmair, Lisbeth Dahl, Øyvind Lie, Marian Kjellevold
BMC Medicine.2018;[Epub] CrossRef - Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: An advancement in metal phytoremediation technology
Shatrohan Lal, Sheel Ratna, Olfa Ben Said, Rajesh Kumar
Environmental Technology & Innovation.2018; 10: 243. CrossRef - C. elegans as a model in developmental neurotoxicology
Joanna A. Ruszkiewicz, Adi Pinkas, Mahfuzur R. Miah, Rebecca L. Weitz, Michael J.A. Lawes, Ayodele J. Akinyemi, Omamuyovwi M. Ijomone, Michael Aschner
Toxicology and Applied Pharmacology.2018; 354: 126. CrossRef - Long- and short-term effects of mercury pollution on the soil microbiome
Aline Frossard, Jonathan Donhauser, Adrien Mestrot, Sebastien Gygax, Erland Bååth, Beat Frey
Soil Biology and Biochemistry.2018; 120: 191. CrossRef - Major global changes interact to cause male-biased sex ratios in a reptile with temperature-dependent sex determination
M.M. Thompson, B.H. Coe, R.M. Andrews, D.F. Stauffer, D.A. Cristol, D.A. Crossley, W.A. Hopkins
Biological Conservation.2018; 222: 64. CrossRef - Hippocampal Dysfunction Provoked by Mercury Chloride Exposure: Evaluation of Cognitive Impairment, Oxidative Stress, Tissue Injury and Nature of Cell Death
Walessa Alana Bragança Aragão, Francisco Bruno Teixeira, Nathalia Carolina Fernandes Fagundes, Rafael Monteiro Fernandes, Luanna Melo Pereira Fernandes, Márcia Cristina Freitas da Silva, Lilian Lund Amado, Fernanda Espírito Santo Sagica, Edivaldo Herculan
Oxidative Medicine and Cellular Longevity.2018; 2018: 1. CrossRef - Electrochemical Hg2+detection at tannic acid-gold nanoparticle modified electrodes by square wave voltammetry
Alex L. Suherman, Sabine Kuss, Eden E. L. Tanner, Neil P. Young, Richard G. Compton
The Analyst.2018; 143(9): 2035. CrossRef - Are the existing guideline values adequate to protect soil health from inorganic mercury contamination?
Khandaker Rayhan Mahbub, Md Mezbaul Bahar, Mallavarapu Megharaj, Maurizio Labbate
Environment International.2018; 117: 10. CrossRef - Unravelling motor behaviour hallmarks in intoxicated adolescents: methylmercury subtoxic-dose exposure and binge ethanol intake paradigm in rats
Aline Nascimento Oliveira, Alana Miranda Pinheiro, Ivaldo Jesus Almeida Belém-Filho, Luanna Melo Pereira Fernandes, Sabrina Carvalho Cartágenes, Paula Cardoso Ribera, Enéas Andrade Fontes-Júnior, Maria Elena Crespo-Lopez, Marta Chagas Monteiro, Marcelo Ol
Environmental Science and Pollution Research.2018; 25(22): 21937. CrossRef - Assessment of human health risk associated with methylmercury in the imported fish marketed in the Caribbean
Fabio Fuentes-Gandara, Claudia Herrera-Herrera, José Pinedo-Hernández, José Marrugo-Negrete, Sergi Díez
Environmental Research.2018; 165: 324. CrossRef - Methylmercury and diphenyl diselenide interactions in Drosophila melanogaster: effects on development, behavior, and Hg levels
Mayara B. Leão, Paulo C. C. da Rosa, Caroline Wagner, Thiago H. Lugokenski, Cristiane L. Dalla Corte
Environmental Science and Pollution Research.2018; 25(22): 21568. CrossRef - Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon
Claudia M. Vega, Jesem D.Y. Orellana, Marcos W. Oliveira, Sandra S. Hacon, Paulo C. Basta
International Journal of Environmental Research and Public Health.2018; 15(6): 1051. CrossRef - Exposure to Inorganic Mercury Causes Oxidative Stress, Cell Death, and Functional Deficits in the Motor Cortex
Francisco B. Teixeira, Ana C. A. de Oliveira, Luana K. R. Leão, Nathália C. F. Fagundes, Rafael M. Fernandes, Luanna M. P. Fernandes, Márcia C. F. da Silva, Lilian L. Amado, Fernanda E. S. Sagica, Edivaldo H. C. de Oliveira, Maria E. Crespo-Lopez, Cristia
Frontiers in Molecular Neuroscience.2018;[Epub] CrossRef - Toxic metal(loid)-based pollutants and their possible role in autism spectrum disorder
Geir Bjørklund, Anatoly V. Skalny, Md. Mostafizur Rahman, Maryam Dadar, Heba A. Yassa, Jan Aaseth, Salvatore Chirumbolo, Margarita G. Skalnaya, Alexey A. Tinkov
Environmental Research.2018; 166: 234. CrossRef - Development of waste-derived sorbents from biomass and brominated flame retarded plastic for elemental mercury removal from coal-fired flue gas
Yang Xu, Fangfang Deng, Qicong Pang, Shuangwu He, Yongqing Xu, Guangqian Luo, Hong Yao
Chemical Engineering Journal.2018; 350: 911. CrossRef - Acute respiratory syndrome following accidental inhalation of mercury vapor
Jorge Cortes, Jaime Peralta, Rienzi Díaz‐Navarro
Clinical Case Reports.2018; 6(8): 1535. CrossRef - Assessing mercury intoxication in isolated/remote populations: Increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon
Gabriela de Paula Fonseca Arrifano, Rosa Del Carmen Rodriguez Martin-Doimeadios, María Jiménez-Moreno, Marcus Augusto-Oliveira, José Rogério Souza-Monteiro, Ricardo Paraense, Camila Rodrigues Machado, Marcelo Farina, Barbarella Macchi, José Luiz Martins d
NeuroToxicology.2018; 68: 151. CrossRef - Development of a novel and robust microprecipitation approach using cetyltrimethyl ammonium bromide (CTAB) for preconcentration and speciation of mercury in waters prior to CVAAS determination
V. Balarama Krishna Mullapudi, Nidhi Garg, Dheeram Karunasagar
International Journal of Environmental Analytical Chemistry.2018; 98(9): 811. CrossRef - Urinary Mercury Levels Among Workers in E-waste Shops in Nakhon Si Thammarat Province, Thailand
Somsiri Decharat
Journal of Preventive Medicine and Public Health.2018; 51(4): 196. CrossRef - Ratio of Mercury Concentration to PCB Concentration Varies with Sex of White Sucker (Catostomus commersonii)
Charles Madenjian, Andrew Stevens, Martin Stapanian, David Krabbenhoft, John DeWild, Jacob Ogorek, William Edwards, Lynn Ogilvie, Peter McIntyre
Environments.2018; 5(9): 94. CrossRef - Design, Development, and Assessment of Lake Water Purification System Through Solar Concentration Technique
Charan Nallode, Rakesh C, Ganesha Prasad M S, Adhvaith M, Ravi Kumar
Water Conservation Science and Engineering.2018; 3(4): 321. CrossRef - Efficient “turn-on” nanosensor by dual emission-quenching mechanism of functionalized Se doped ZnO nanorods for mercury (II) detection
A. V. R. Krishna Rao, Ramesh B. Reddy, Sagnik Sengupta, Venkatesh Chelvam
Applied Nanoscience.2018; 8(8): 1973. CrossRef - Percentage of methylmercury in the muscle tissue of freshwater fish varies with body size and age and among species
Gretchen L. Lescord, Thomas A. Johnston, Brian A. Branfireun, John M. Gunn
Environmental Toxicology and Chemistry.2018; 37(10): 2682. CrossRef - Variation in thyroid hormone levels is associated with elevated blood mercury levels among artisanal small-scale miners in Ghana
Justice Afrifa, Wisdom Djange Ogbordjor, Ruth Duku-Takyi, Jaymie Meliker
PLOS ONE.2018; 13(8): e0203335. CrossRef - Introducing Greek Guidelines for the Diagnosis and Treatment of Adverse Health Effects of Occupational Exposure to Metals
Evangelia Nena, Sotirios Koupidis, Eleni Douna, Anastasia Kikemenis, Georgios Dounias
Annals of Global Health.2018; 84(3): 470. CrossRef - Mercury and arsenic attenuate canonical and non-canonical NLRP3 inflammasome activation
Huijeong Ahn, Jeongeun Kim, Seung Goo Kang, Sung-il Yoon, Hyun-Jeong Ko, Pyeung-Hyeun Kim, Eui-Ju Hong, Beum-Soo An, Eunsong Lee, Geun-Shik Lee
Scientific Reports.2018;[Epub] CrossRef - Comparison of Reactive Gaseous Mercury Collection by Different Sampling Methods in a Laboratory Test and Field Monitoring
Xiaoge Bu, Hefeng Zhang, Guangkuo Lv, Huiming Lin, Long Chen, Xiufeng Yin, Guofeng Shen, Wen Yuan, Wei Zhang, Xuejun Wang, Yindong Tong
Environmental Science & Technology Letters.2018; 5(10): 600. CrossRef - Natural Phytotherapeutic Antioxidants in the Treatment of Mercury Intoxication-A Review
Velid Unsal
Advanced Pharmaceutical Bulletin.2018; 8(3): 365. CrossRef - Toxicological and nutritional status of trace elements in hair of women with in vitro fertilization (IVF) pregnancy and their 9-month-old children
Anatoly V. Skalny, Alexey A. Tinkov, Tatiana G. Bohan, Marina B. Shabalovskaya, Olga Terekhina, Svetlana B. Leshchinskaia, Lyubov A. Agarkova, Svetlana V. Notova, Margarita G. Skalnaya, Yulia Kovas
Reproductive Toxicology.2018; 82: 50. CrossRef - Total mercury in surficial bottom sediments of Volga River’s reservoirs in Central Russia
Yury G. Udodenko, Viktor T. Komov, Viktor V. Zakonnov
Environmental Earth Sciences.2018;[Epub] CrossRef - Exposure to Mercury in Workers and the Population Surrounding Gold Mining Areas in the Mojana Region, Colombia
Sonia Mireya Díaz, Maria Nathalia Muñoz-Guerrero, Marien Palma-Parra, Carolina Becerra-Arias, Julián Alfredo Fernández-Niño
International Journal of Environmental Research and Public Health.2018; 15(11): 2337. CrossRef - A Review of Metal Exposure and Its Effects on Bone Health
Juliana Rodríguez, Patricia Mónica Mandalunis
Journal of Toxicology.2018; 2018: 1. CrossRef - INFLUENCE OF MERCURY ACCUMULATION
ON THE HEALTH STATUS OF REPRODUCTIVE AGE WOMEN
O.P. Shuvalova, E.S. Ivanova, V.T. Komov
ЗДОРОВЬЕ НАСЕЛЕНИЯ И СРЕДА ОБИТАНИЯ - ЗНиСО / PUBLIC HEALTH AND LIFE ENVIRONMENT.2018; : 36. CrossRef - ASSESSING
CHANGES IN THE QUALITY OF DRINKING WATER WHEN CHANGING OF
WATER SUPPLY SOURCE
G.T. Mirsaitova, V.V. Dolgikh, R.Ya. Khamitova, A.A. Imamov, S.Yu. Filippova
ЗДОРОВЬЕ НАСЕЛЕНИЯ И СРЕДА ОБИТАНИЯ - ЗНиСО / PUBLIC HEALTH AND LIFE ENVIRONMENT.2018; : 31. CrossRef - Congenital poisoning after maternal parenteral mercury administration
Courchia Benjamin
Journal of Advanced Pediatrics and Child Health.2018; 1(1): 001. CrossRef - Toxic effects of mercury on the cell nucleus of Dictyostelium discoideum
Lara Boatti, Fabio Rapallo, Aldo Viarengo, Francesco Marsano
Environmental Toxicology.2017; 32(2): 417. CrossRef - Current progress on understanding the impact of mercury on human health
Eunhee Ha, Niladri Basu, Stephan Bose-O’Reilly, José G. Dórea, Emeir McSorley, Mineshi Sakamoto, Hing Man Chan
Environmental Research.2017; 152: 419. CrossRef - Preconception health behaviours: A scoping review
Kirsti I. Toivonen, Kirsten A. Oinonen, Katelyn M. Duchene
Preventive Medicine.2017; 96: 1. CrossRef - Mercury toxicity to terrestrial biota
Khandaker Rayhan Mahbub, Kannan Krishnan, Ravi Naidu, Stuart Andrews, Mallavarapu Megharaj
Ecological Indicators.2017; 74: 451. CrossRef - Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview
Virginia Andreoli, Francesca Sprovieri
International Journal of Environmental Research and Public Health.2017; 14(1): 93. CrossRef - Occupational mercury vapour poisoning with a respiratory failure, pneumomediastinum and severe quadriparesis
Jakub Smiechowicz, Anna Skoczynska, Agata Nieckula-Szwarc, Katarzyna Kulpa, Andrzej Kübler
SAGE Open Medical Case Reports.2017; 5: 2050313X1769547. CrossRef - The cessation of the long-term exposure to low doses of mercury ameliorates the increase in systolic blood pressure and vascular damage in rats
Danize Aparecida Rizzetti, João Guilherme Dini Torres, Alyne Goulart Escobar, Taiz Martins da Silva, Paola Zambelli Moraes, Raquel Hernanz, Franck Maciel Peçanha, Marta Miguel Castro, Dalton Valentim Vassallo, Mercedes Salaices, Maria Jesús Alonso, Giulia
Environmental Research.2017; 155: 182. CrossRef - Uptake of Pb(II) and Cd(II) on Chitosan Microsphere Surface Successively Grafted by Methyl Acrylate and Diethylenetriamine
Haifeng Zhang, Qifeng Dang, Chengsheng Liu, Dongsu Cha, Zhenzhen Yu, Wenjing Zhu, Bing Fan
ACS Applied Materials & Interfaces.2017; 9(12): 11144. CrossRef - Physiological and biochemical impacts induced by mercury pollution and seawater acidification in Hediste diversicolor
Rosa Freitas, Lucia de Marchi, Anthony Moreira, João L.T. Pestana, Frederick J. Wrona, Etelvina Figueira, Amadeu M.V.M. Soares
Science of The Total Environment.2017; 595: 691. CrossRef - Serum heavy metals and lung function in a chronic obstructive pulmonary disease cohort
Jeongwon Heo, Hyun Sun Park, Yoonki Hong, Jinkyeong Park, Seok-Ho Hong, Chi Young Bang, Myoung-Nam Lim, Woo Jin Kim
Toxicology and Environmental Health Sciences.2017; 9(1): 30. CrossRef - The 9th Conference on Metal Toxicity and Carcinogenesis: The conference overview
James T.F. Wise, Lei Wang, Zhuo Zhang, Xianglin Shi
Toxicology and Applied Pharmacology.2017; 331: 1. CrossRef - Gaseous Elemental Mercury and Total and Leached Mercury in Building Materials from the Former Hg-Mining Area of Abbadia San Salvatore (Central Italy)
Orlando Vaselli, Barbara Nisi, Daniele Rappuoli, Jacopo Cabassi, Franco Tassi
International Journal of Environmental Research and Public Health.2017; 14(4): 425. CrossRef - Mercury levels in parturient and newborns from Aveiro region, Portugal
Ana Catarina Alves, Marta S. Monteiro, Ana Luísa Machado, Mário Oliveira, Ana Bóia, Ana Correia, Nuno Oliveira, Amadeu M.V.M. Soares, Susana Loureiro
Journal of Toxicology and Environmental Health, Part A.2017; 80(13-15): 697. CrossRef - Mercury levels of yellowfin tuna (Thunnus albacares) are associated with capture location
Sascha C.T. Nicklisch, Lindsay T. Bonito, Stuart Sandin, Amro Hamdoun
Environmental Pollution.2017; 229: 87. CrossRef - Poly(benzoxazine‐co‐sulfur): An efficient sorbent for mercury removal from aqueous solution
Sema Akay, Berkant Kayan, Dimitrios Kalderis, Mustafa Arslan, Yusuf Yagci, Baris Kiskan
Journal of Applied Polymer Science.2017;[Epub] CrossRef - Assessment of Heavy Metal Concentrations in Pawpaw (Carica papaya Linn.) around Automobile Workshops in Port Harcourt Metropolis, Rivers State, Nigeria
Olatunde Sunday Eludoyin, Onisoya Margaret Ogbe
Journal of Health and Pollution.2017; 7(14): 48. CrossRef - Enhancing performances of colorimetric response of carboxymethylcellulose-stabilized silver nanoparticles: A fully eco-friendly assay for Hg2+ detection
Nawfel Sakly, Wiem Marzouk, Hafedh Ben Ouada, Hatem Majdoub
Sensors and Actuators B: Chemical.2017; 253: 918. CrossRef - Mortality and transcriptional effects of inorganic mercury in the marine copepod Calanus finmarchicus
Knut Erik Tollefsen, You Song, Tore Høgåsen, Ida Beathe Øverjordet, Dag Altin, Bjørn Henrik Hansen
Journal of Toxicology and Environmental Health, Part A.2017; 80(16-18): 845. CrossRef - Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation
W Liu, Z Xu, H Li, M Guo, T Yang, S Feng, B Xu, Yu Deng
Human & Experimental Toxicology.2017; 36(9): 949. CrossRef - Methylmercury exposure for 14 days (short-term) produces behavioral and biochemical changes in mouse cerebellum, liver, and serum
Sérgio José Macedo-Júnior, Murilo Luiz-Cerutti, Denise B. Nascimento, Marcelo Farina, Adair Roberto Soares Santos, Alcíbia Helena de Azevedo Maia
Journal of Toxicology and Environmental Health, Part A.2017; 80(19-21): 1145. CrossRef - Risk assessment of environmental exposure to heavy metals in mothers and their respective infants
Iman Al-Saleh, Reem Al-Rouqi, Rola Elkhatib, Mai Abduljabbar, Tahreer Al-Rajudi
International Journal of Hygiene and Environmental Health.2017; 220(8): 1252. CrossRef - Air Pollution and Public Health: A PRISMA-Compliant Systematic Review
Marco Quarato, Luigi De Maria, Maria Gatti, Antonio Caputi, Francesca Mansi, Pietro Lorusso, Francesco Birtolo, Luigi Vimercati
Atmosphere.2017; 8(10): 183. CrossRef - Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction
Sally Sabra, Ebba Malmqvist, Alicia Saborit, Eduard Gratacós, Maria Dolores Gomez Roig, Rogelio Cruz-Martinez
PLOS ONE.2017; 12(10): e0185645. CrossRef - Insights into the origin of the excited transitions in graphene quantum dots interacting with heavy metals in different media
Ivan Shtepliuk, Volodymyr Khranovskyy, Rositsa Yakimova
Physical Chemistry Chemical Physics.2017; 19(45): 30445. CrossRef - Recent Studies on the Speciation and Determination of Mercury in Different Environmental Matrices Using Various Analytical Techniques
Lakshmi Narayana Suvarapu, Sung-Ok Baek
International Journal of Analytical Chemistry.2017; 2017: 1. CrossRef - Rat spinal ganglia in assessment of protective action of antioxidants: A morphological study
Liudmyla M. Sokurenko, Mariya O. Savchyna, Viktor I. Litus, Rostyslav F. Kaminsky, Yurii B. Сhaikovsky
Medicina.2017; 53(5): 316. CrossRef - Low-level mercury, omega-3 index and neurobehavioral outcomes in an adult US coastal population
Caterina Vacchi-Suzzi, Roxanne Karimi, Danielle Kruse, Susan M. Silbernagel, Keith E. Levine, Diane S. Rohlman, Jaymie R. Meliker
European Journal of Nutrition.2016; 55(2): 699. CrossRef - Evaluation of mercury, cadmium and lead levels in fish and fishery products imported by air in North Italy from extra-European Union Countries
Cristina Galimberti, Ivan Corti, Massimo Cressoni, Vittorio M. Moretti, Simonetta Menotta, Umberto Galli, Donatella Cambiaghi
Food Control.2016; 60: 329. CrossRef - A review on the distribution of Hg in the environment and its human health impacts
Ki-Hyun Kim, Ehsanul Kabir, Shamin Ara Jahan
Journal of Hazardous Materials.2016; 306: 376. CrossRef - Structural and Functional Responses of Macrobenthic Communities to Mercury Contamination
P. Matos, E. Sousa, M. A. Pardal, E. Pereira, P. G. Cardoso
Water, Air, & Soil Pollution.2016;[Epub] CrossRef - Influence of metal(loid) bioaccumulation and maternal transfer on embryo-larval development in fish exposed to a major coal ash spill
Mark S. Greeley, S. Marshall Adams, Logan R. Elmore, Mary K. McCracken
Aquatic Toxicology.2016; 173: 165. CrossRef - Hydroxytyrosol inhibits phosphatidylserine exposure and suicidal death induced by mercury in human erythrocytes: Possible involvement of the glutathione pathway
Arbace Officioso, Kousi Alzoubi, Florian Lang, Caterina Manna
Food and Chemical Toxicology.2016; 89: 47. CrossRef - Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids
Jennifer Watts
Journal of Clinical Medicine.2016; 5(2): 19. CrossRef - Contaminants alimentaires et le risque de cancer
Xavier Coumoul
Cahiers de Nutrition et de Diététique.2016; 51(2): 104. CrossRef - Results of micronucleus assays with individuals who are occupationally and environmentally exposed to mercury, lead and cadmium
Armen Nersesyan, Michael Kundi, Monika Waldherr, Tahereh Setayesh, Miroslav Mišík, Georg Wultsch, Metka Filipic, Gustavo Rafael Mazzaron Barcelos, Siegfried Knasmueller
Mutation Research/Reviews in Mutation Research.2016; 770: 119. CrossRef - Mercury as a possible link between maternal obesity and autism spectrum disorder
Anatoly V. Skalny, Margarita G. Skalnaya, Geir Bjørklund, Alexandr A. Nikonorov, Alexey A. Tinkov
Medical Hypotheses.2016; 91: 90. CrossRef - In situ generation of functionalized 1,2,4-triazole-5-thiones containing pyridine or pyrazine moieties and their coordination to HgII
Elena Bermejo, Alfonso Castiñeiras, Isabel García-Santos, Raúl Rodríguez-Riobó
CrystEngComm.2016; 18(19): 3428. CrossRef - Ototoxicity of Divalent Metals
Jerome A. Roth, Richard Salvi
Neurotoxicity Research.2016; 30(2): 268. CrossRef - Combined toxicity of silica nanoparticles and methylmercury on cardiovascular system in zebrafish (Danio rerio) embryos
Junchao Duan, Hejing Hu, Qiuling Li, Lizhen Jiang, Yang Zou, Yapei Wang, Zhiwei Sun
Environmental Toxicology and Pharmacology.2016; 44: 120. CrossRef - Coastal erosion as a source of mercury into the marine environment along the Polish Baltic shore
Magdalena Bełdowska, Agnieszka Jędruch, Leszek Łęczyński, Dominika Saniewska, Urszula Kwasigroch
Environmental Science and Pollution Research.2016; 23(16): 16372. CrossRef - Inorganic mercury exposure in drinking water alters essential metal homeostasis in pregnant rats without altering rat pup behavior
Cláudia S. Oliveira, Vitor A. Oliveira, Lidiane M. Costa, Taíse F. Pedroso, Mariana M. Fonseca, Jamile S. Bernardi, Tiago L. Fiuza, Maria E. Pereira
Reproductive Toxicology.2016; 65: 18. CrossRef - The risk of high mercury accumulation in edible mushrooms cultivated on contaminated substrates
Piotr Rzymski, Mirosław Mleczek, Marek Siwulski, Monika Gąsecka, Przemysław Niedzielski
Journal of Food Composition and Analysis.2016; 51: 55. CrossRef - Ecological Risk Assessment of Metals Contamination in the Sediments of Natural Urban Wetlands in Dry Tropical Climate
Vivek Rana, Subodh Kumar Maiti, Sheeja Jagadevan
Bulletin of Environmental Contamination and Toxicology.2016; 97(3): 407. CrossRef - The organic mercury compounds, methylmercury and ethylmercury, inhibited ciliary movement of ventricular ependymal cells in the mouse brain around the concentrations reported for human poisoning
Shigeru Yoshida, Shinsaku Matsumoto, Takuya Kanchika, Teruki Hagiwara, Takeshi Minami
NeuroToxicology.2016; 57: 69. CrossRef - Cyanidation of Mercury-Contaminated Tailings: Potential Health Effects and Environmental Justice
Kevin Drace, Adam M. Kiefer, Marcello M. Veiga
Current Environmental Health Reports.2016; 3(4): 443. CrossRef - Mercury Exposure in Children of the Wanshan Mercury Mining Area, Guizhou, China
Buyun Du, Ping Li, Xinbin Feng, Guangle Qiu, Jun Zhou, Laurence Maurice
International Journal of Environmental Research and Public Health.2016; 13(11): 1107. CrossRef - Non-occupational exposure to heavy metals of the residents of an industrial area and biomonitoring
Luigi Vimercati, Antonio Baldassarre, Maria F Gatti, Tommaso Gagliardi, Maria Serinelli, Luigi De Maria, Antonio Caputi, Angelica A Dirodi, Ida Galise, Francesco Cuccaro, Giorgio Assennato
Environmental Monitoring and Assessment.2016;[Epub] CrossRef - Regulation of Sirt1/Nrf2/TNF-α signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity
Daqian Yang, Xiao Tan, Zhanjun Lv, Biying Liu, Ruiqi Baiyun, Jingjing Lu, Zhigang Zhang
Scientific Reports.2016;[Epub] CrossRef - Effects on and transfer across the blood-brain barrier in vitro—Comparison of organic and inorganic mercury species
Hanna Lohren, Julia Bornhorst, Romy Fitkau, Gabriele Pohl, Hans-Joachim Galla, Tanja Schwerdtle
BMC Pharmacology and Toxicology.2016;[Epub] CrossRef - Influence of environmental factors in the development of inflammatory bowel diseases
Evangelia Legaki
World Journal of Gastrointestinal Pharmacology and Therapeutics.2016; 7(1): 112. CrossRef - Metals and Neurodegeneration
Pan Chen, Mahfuzur Rahman Miah, Michael Aschner
F1000Research.2016; 5: 366. CrossRef - Prediction of embryotoxic potential using the ReProGlo stem cell-based Wnt reporter assay
Frederik Uibel, Michael Schwarz
Reproductive Toxicology.2015; 55: 30. CrossRef - Blood Mercury Can Be a Factor of Elevated Serum Ferritin: Analysis of Korea National Health and Nutrition Examination Survey (KNHANES 2008–2012)
Nam-Seok Joo, Young-Hwa Choi, Kyung-Jin Yeum, Soo-Jung Park, Beomhee Choi, Young-Sang Kim
Biological Trace Element Research.2015; 164(1): 3. CrossRef - Mercury in soil profiles from metal mining and smelting areas in Namibia and Zambia: distribution and potential sources
Filip Podolský, Vojtěch Ettler, Ondřej Šebek, Josef Ježek, Martin Mihaljevič, Bohdan Kříbek, Ondra Sracek, Aleš Vaněk, Vít Penížek, Vladimír Majer, Ben Mapani, Fred Kamona, Imasiku Nyambe
Journal of Soils and Sediments.2015; 15(3): 648. CrossRef - Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions
Siong Luong Ting, Shu Jing Ee, Arundithi Ananthanarayanan, Kam Chew Leong, Peng Chen
Electrochimica Acta.2015; 172: 7. CrossRef - Prenatal mercury concentration is associated with changes in DNA methylation atTCEANC2in newborns
Kelly M Bakulski, HwaJin Lee, Jason I Feinberg, Ellen M Wells, Shannon Brown, Julie B Herbstman, Frank R Witter, Rolf U Halden, Kathleen Caldwell, Mary Ellen Mortensen, Andrew E Jaffe, John Moye, Laura E Caulfield, Yi Pan, Lynn R Goldman, Andrew P Feinber
International Journal of Epidemiology.2015; 44(4): 1249. CrossRef - The protective role of olive oil hydroxytyrosol against oxidative alterations induced by mercury in human erythrocytes
Lidia Tagliafierro, Arbace Officioso, Sergio Sorbo, Adriana Basile, Caterina Manna
Food and Chemical Toxicology.2015; 82: 59. CrossRef - Electrochemical monitoring of decolorization of diazo dye Evans blue by Fenton process under anaerobic conditions: Kinetics and optimization
Cabir Arat, Ender Biçer
Russian Journal of Electrochemistry.2015; 51(8): 730. CrossRef - Mercury and Selenium in Muscle and Target Organs of Scalloped Hammerhead Sharks Sphyrna lewini of the SE Gulf of California: Dietary Intake, Molar Ratios, Loads, and Human Health Risks
Magdalena E. Bergés-Tiznado, Fernando Márquez-Farías, Raúl E. Lara-Mendoza, Yassir E. Torres-Rojas, Felipe Galván-Magaña, Humberto Bojórquez-Leyva, Federico Páez-Osuna
Archives of Environmental Contamination and Toxicology.2015; 69(4): 440. CrossRef - High mercury seafood consumption associated with fatigue at specialty medical clinics on Long Island, NY
Shivam Kothari, Danielle Kruse, Roxanne Karimi, Susan Silbernagel, Nurcan Gursoy, Raja Jaber, Heidi Roppelt, Rina Awan, Avram Gold, Jaymie R. Meliker
Preventive Medicine Reports.2015; 2: 798. CrossRef - The blood–cerebrospinal fluid barrier – first evidence for an active transport of organic mercury compounds out of the brain
Hanna Lohren, Julia Bornhorst, Hans-Joachim Galla, Tanja Schwerdtle
Metallomics.2015; 7(10): 1420. CrossRef - Stability and Performance of Physically Immobilized Ionic Liquids for Mercury Adsorption from a Gas Stream
Tauqeer Abbas, Lethesh Kallidanthiyil Chellappan, M. I Abdul Mutalib, Kuah Yong Cheun, Syed Nasir Shah, Salman Nazir, Amiruddin Hassan, Mahpuzah bt Abai, Eakalak Khan
Industrial & Engineering Chemistry Research.2015; 54(48): 12114. CrossRef - Mycoplasmosis and upper respiratory tract disease of tortoises: A review and update
Elliott R. Jacobson, Mary B. Brown, Lori D. Wendland, Daniel R. Brown, Paul A. Klein, Mary M. Christopher, Kristin H. Berry
The Veterinary Journal.2014; 201(3): 257. CrossRef - Evaluation of the Effects of Chronic Intoxication with Inorganic Mercury on Memory and Motor Control in Rats
Francisco Teixeira, Rafael Fernandes, Paulo Farias-Junior, Natacha Costa, Luanna Fernandes, Luana Santana, Ademir Silva-Junior, Marcia Silva, Cristiane Maia, Rafael Lima
International Journal of Environmental Research and Public Health.2014; 11(9): 9171. CrossRef
 KSPM
KSPM

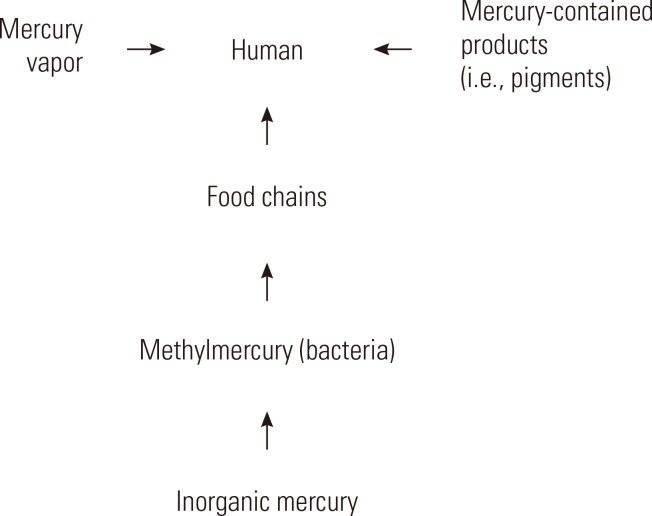
 PubReader
PubReader ePub Link
ePub Link Cite
Cite


