Pre-pregnancy Diet to Maternal and Child Health Outcome: A Scoping Review of Current Evidence
Article information
Abstract
Objectives
Pre-pregnancy diet has an important role in preparing for healthy generation. However, evidence on this issue has been scarce. A scoping review synthesising current evidence will support the demand to map ‘what has been researched’ on pre-pregnancy diet and maternal and child health.
Methods
Systematic search was performed using PICOS (Population, Intervention, Comparison, Outcomes, and Study design) framework in electronic databases. Articles were screened for eligibility, summarized, and the quality was assessed using the National Institute of Health assessment tool. The review structure complies with Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews guide.
Results
Forty-two articles were included after full-text screening. Twenty-five studies were in high-income countries (HICs), six in each upper-middle income, five in lower-middle income countries (LMICs), and one in low-income countries (LIC). Based on the regions: North America (n=16), Europe (n=5), South America (n=4), Australia (n=4), Asia (n=5), Middle East (n=2), and sub-Saharan Africa (n=1). The two-most observed diet-related exposures were dietary pattern (n=17) and dietary quality (n=12). The most assessed outcome was gestational diabetes mellitus (n=28) and fetal and newborn anthropometry (n=7). The average quality score±standard deviation was 70±18%.
Conclusions
Research related to pre-pregnancy diet is still concentrated in HICs. The context of diet may vary; therefore, future research is encouraged in LMICs and LICs context, and Mediterranean, South-East Asia, Pacific, and African regions. Some maternal and child nutrition-related morbidity, such as anemia and micronutrient deficiencies, have not been discussed. Research on these aspects will benefit to fill in the gaps related to pre-pregnancy diet and maternal and child health.
INTRODUCTION
Pre-pregnancy or preconception health, not until recently, has come to attention as a window of opportunity to prepare for a healthy pregnancy. Pregnancy itself is not always a prepared event. In the United Kingdom, 45% of pregnancies were unplanned at the time of conception [1], and the rate may be even higher in developing countries with less access to family planning. Preconception health includes a wide range of nutrition and lifestyle aspects, one of which is diet. Healthy diets contribute to preventing malnutrition, and in the long term, reducing the risk of diet-related non-communicable diseases (NCDs) [2]. Studies have observed the associations between pre-pregnancy lifestyle and maternal and child health outcomes [3]. Evidence also suggests that women’s nutritional status before pregnancy is associated with maternal and child outcomes [4]. However, the role of diet before pregnancy in the development of maternal and child outcomes is not much discussed.
Understanding the role of pre-pregnancy diet in maternal and child health will contribute to maternal and child morbidity and mortality as well as NCDs prevention. Nutritional status is directly influenced by dietary intake, whereas, a healthy diet contributes to macronutrient and micronutrient adequacy and balanced energy [5]. In high-income countries (HICs), child-bearing age women’s diet typically had a high intake of refined sugar and a high-fat diet, but low intakes of fruits, vegetables, and protein source food [3]. The national surveys in some HICs also revealed that the young adult age group have also been reported to have lower than recommended intakes for iodine, iron, and folate, which are important for pregnancy [3,6]. Therefore, it raises concerns about nutritional preparedness in pregnancy.
Research related to the pre-pregnancy diet are highly diverse. The context of diet is widely affected by socio-cultural, geographical, and economy. The maternal and child health constraints also vary depending on the region and country’s socioeconomic level, leading to the possible differences in the evidence-based application based on regional context. Information related to what evidence is available, what outcomes already measured, and in what context, will support the demand for more evidence on pre-pregnancy diet’s role in future health. This scoping review aims to explore the current evidence of diet in the pre-pregnancy stage to the maternal and child health outcome. A preliminary search of PROSPERO, MEDLINE, the Cochrane Database of Systematic Reviews, and JBI Evidence Synthesis was conducted and no current or in-progress scoping reviews or systematic reviews on the topic were identified. To answer the question of: What have been done in the existing research of pre-pregnancy diet as exposure to maternal and child health outcome?, this scoping review systematically maps the available evidence and identify the gaps for further research.
METHODS
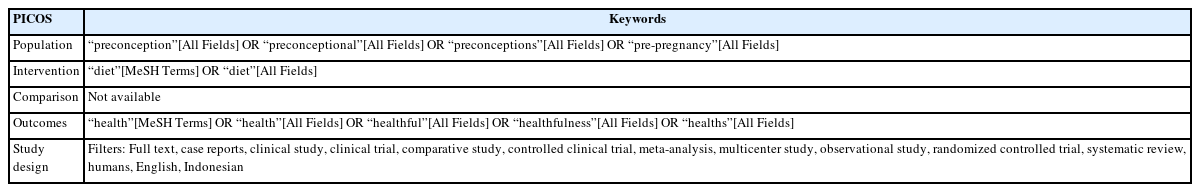
This scoping review was structured according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews guide for scoping review. The literature search was performed using PICOS (Population, Intervention, Comparison, Outcomes, and Study design) framework (Table 1). The use of PICOS was chosen as the framework includes the necessary observation and more sensitivity for a scoping review related to quantitative health research compared to other frameworks, such as PEOS, SPICE, SPIDER, and others. PICOS framework also provides a comprehensive search that benefits research with limited time and resources [7]. The topic to adhere to was pre-pregnancy or preconception diet and its relation to maternal and/or child health-related outcomes. Pre-pregnancy was defined as the time before conception or pregnancy, which includes the phase in preparing for pregnancy as well as the adolescent period. The diet variable may include nutritional content, food-based supplementation, dietary quality, dietary pattern, and other dietary-related variables, however, did not include non-food-based supplementation and nutritional status. Maternal health was defined as the health-related condition of the mother during the pregnancy, childbirth, and post-natal period [8], therefore did not include the fertility outcome variable. Child health was considered as all aspects of childhood illness starting from birth. Maternal and child health search were combined as a ‘health’ term due to the wider scope of search compared to the use of separated ‘maternal’ and ‘child’ terms. Further selection was conducted in the article identification process.
The Article Identification
We conducted a search through some potential bibliographic databases in July 2022. The systematic search was conducted in PubMed, the largest electronic medical bibliography. The search strategy based on the PICOS framework resulted in the use of the following concept filters: (1) pre-pregnancy or preconception; (2) diet; and (3) health. The final search strategy is available in Supplemental Material 1. An additional systematic search using keywords combination of “Pre-pregnancy diet”, “Prepregnancy diet”, “Pre-conception diet”, “Preconception diet”, and “maternal health” and “child health” was also conducted through PubMed to identify more related articles. Articles were filtered for publication in full text in Indonesian or English languages, involving human participants, and article type based on PubMed NCBI filters: reviews, case reports, clinical study, clinical trial, comparative study, controlled clinical trial, multicentre study, observational study, randomized controlled trial (RCT), systematic review, and meta-analysis. Another search attempt was conducted through Indonesia research bibliography; SINTA; Indonesian Scientific Journal Database; and Google Scholar using local keywords for pre-pregnancy diet; “Pola makan prahamil“; “Pola makan prakonsepsi”; and “Pola makan prakehamilan”. Only peer-reviewed articles were included.
To be included, the article needed to observe pre-pregnancy diet as exposure and maternal and/or child health as the outcome. The article should be peer-reviewed original article, involving human participants, and available in full-text. Articles that are methodological paper, pre-prints, conference article, and in language that was not understood by the authors (aside from English and Bahasa Indonesia) were excluded. The list of inclusion and exclusion criteria can also be found in Supplemental Material 2.
The Selection of Relevant Articles
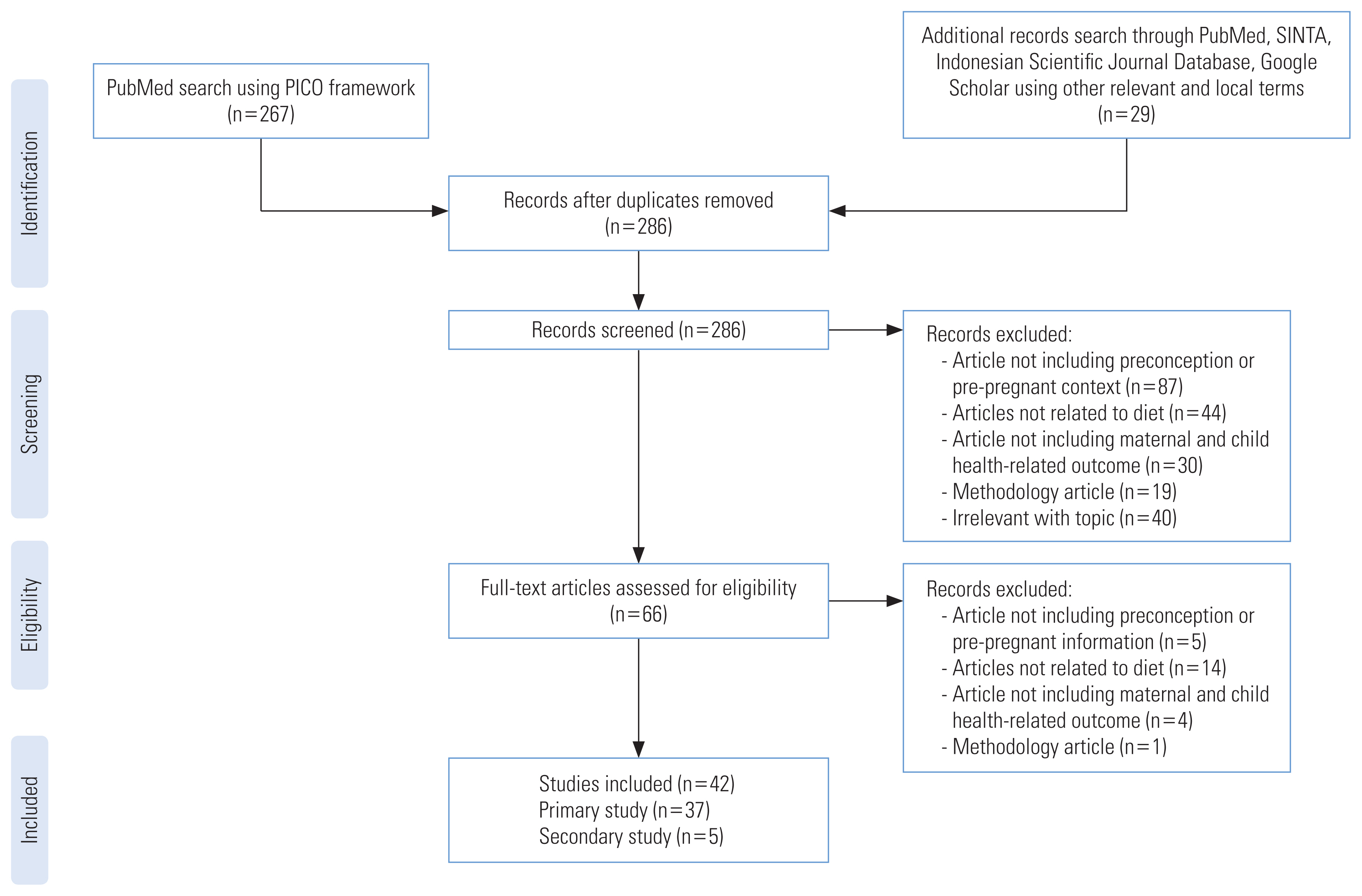
After the articles were cleaned for duplicates, two authors (FW and DGAY), with sufficient capacity to understand English scientific article, each screened for the article separately. The articles were screened by title and abstract for adherence to the topic. The authors then converged their selection of articles to be assessed further for eligibility. Full-text articles were assessed separately by the authors. The final inclusion of the articles in the scoping review was based on the authors’ mutual agreement on the article’s adherence to the topic. In the condition when the authors did not reach a consensus, AG was consulted. The article selection flow was as presented in Figure 1.
Data Extraction and Quality Appraisal
After the final article selection, the studies were grouped into primary research (case reports, clinical study, clinical trial, comparative study, controlled clinical trial, multicentre study, observational study, and RCT) and secondary research (review, systematic review, and meta-analysis). The following information was recorded using a Microsoft Excel spreadsheet for primary research; author(s); year of publication; year of data collection; study design; study group (if any); population; sample size; subject’s age group; country; country’s income level; observed or intervention group; outcome variable; time of the assessed diet; dietary assessment method (if any); maternal health finding(s); and child health finding(s). The time of the assessed diet represents the time frame in which the participant is required to recall or report. As for secondary researchs, the following information were recorded; author(s); year of publication; study design; sample size; exposure variable; outcome variable; maternal health finding(s); and child health finding(s). We also summarized the observed maternal and child health outcome for each diet-related variable.
To provide better insight in the currently available evidence, the quality of the articles were appraised based on the National Institute of Health study quality assessment tool [9] with the FW and DY as the Raters. The overall article’s quality was decided by the average percentage of ‘Yes’ answers on the study quality assessment tools given by the Raters. When no consensus reached by the Raters, AG was consulted. We grouped the article’s quality ratings into three groups; poor (fulfilling less than 33.3% of the criteria); fair (fulfilling 33.3 to 66.6% of the criteria); good (fulfilling more than 66.6% of the criteria). Quality assessment of articles were conducted to evaluate the current quality of evidence available in this review to map the currently available study’s quality, not as a basis of inclusion.
Protocol Registration
This scoping review protocol has been registered in the Open Science Framework Registries: https://doi.org/10.17605/OSF.IO/7F9C2.
Ethics Statement
This study does not include human subject involvement, therefore exempted for institutional review board approval.
RESULTS
The article identification and selection are presented in Figure 1. A search using the PICOS framework in the PubMed database was conducted and yielded 267 articles after filtering for full-text, language, and types of articles. An additional 29 articles were obtained from linked research, database, and the web. After cleaning for duplicates, 286 articles were screened for adherence to the topic. A total of 42 articles were included after the full-text screening, consisting of 37 primary research articles and five secondary research articles to be included in the current scoping review.
The Characteristics of the Studies
The extracted findings from the included primary research were as shown in Table 2 [10–46]. One study is cross-sectional, three are case-control, one is a post-hoc observational study, 27 are cohort, and five studies are RCT. All of the primary studies were quantitative. Out of 42 included studies, 27 of them are based on big projects, mostly based on the Nurses’ Health Study II cohort (13 studies). This scoping review did not use a limiter of date of publication in the search process. However, studies included in the final selection were published in 2009 to 2022.
Of the 42 studies included, 25 studies were conducted in high-income countries (HICs), six in upper-middle-income countries (UMICs), five in lower-middle-income countries (LMICs), and only one was conducted in low-income country (LIC). Based on the geographical areas, 16 were conducted in North America, five were in Europe, four were in South America, four were in Australia, four were in East and South Asia, two were in the Middle East, one in South-East Asia, and one was in sub-Saharan Africa. The proportion of observed pre-pregnancy diet variables based on region was presented in Figure 2A. The pre-pregnancy dietary patterns had been observed in almost all regions but sub-Saharan Africa and East and South Asia. Meanwhile, pre-pregnancy dietary quality research had only been brought up in regions with UMICs and HICs, without any known studies of pre-pregnancy dietary quality from Asia, Middle East, and sub-Saharan Africa. The diet variable as the exposure was mostly (30 studies) measured by food frequency questionnaire (FFQ), other methods used were 24-hour dietary recall, questionnaire, and interview. A total of 13 studies were conducted in the already pregnant population and rely on recall of the diet during the pre-pregnancy phase. Figure 2B shows the type of diet-related variables that were considered as exposures based on the included studies and its maternal and child health outcome variable. The duration of observation from pre-pregnancy to outcome were varied as seen in Figure 2C. The shortest and the most common observation, 15 studies were started no more than six months before pregnancy to observe the outcomes during pregnancy. The longest observation was until adolescent by only one study.
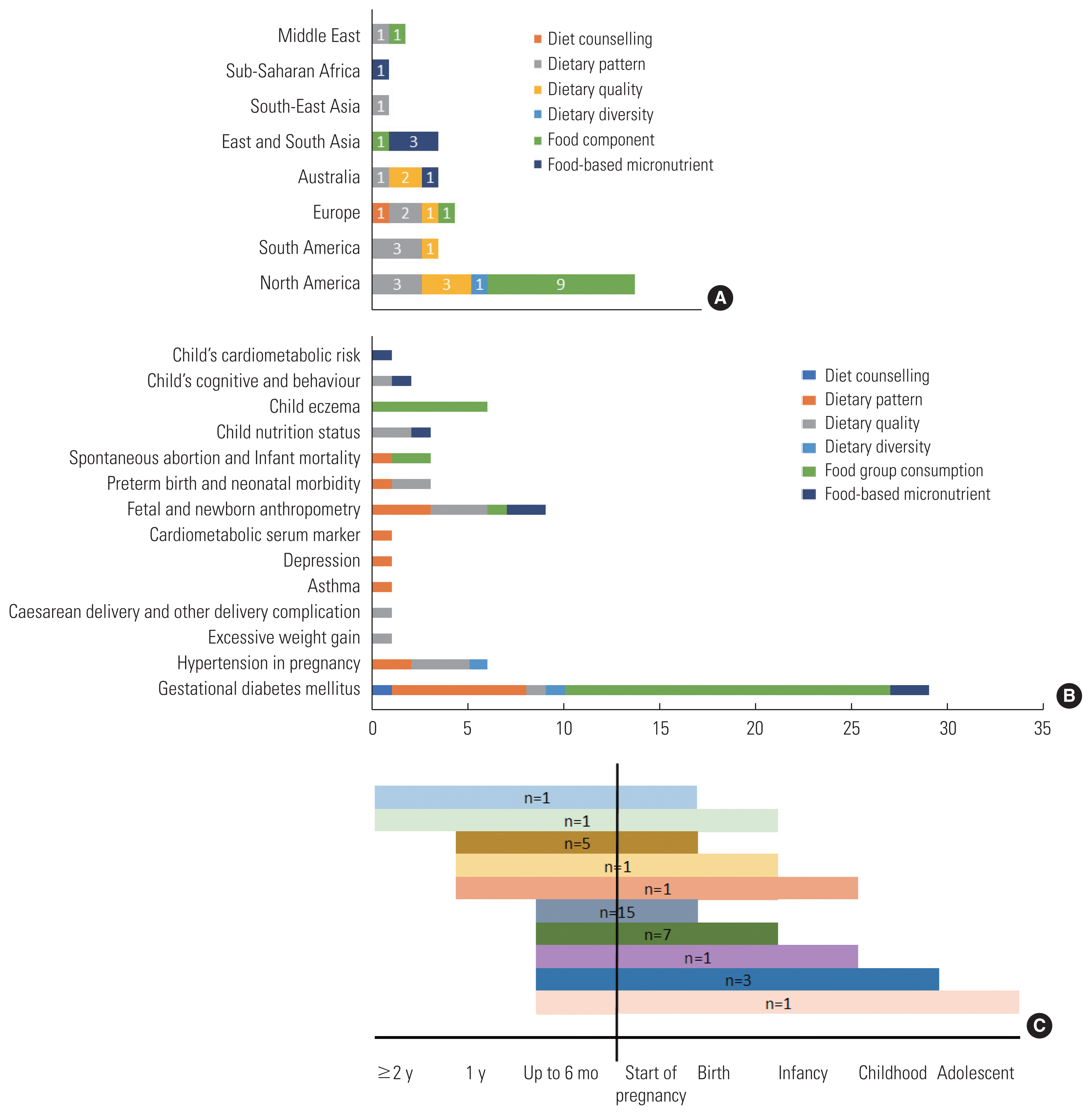
(A) Pre-pregnancy diet research variable theme based on regions; (B) Pre-pregnancy diet research variable theme based on observed outcomes; (C) Timeline of the available studies.
Table 3 [47–51] presents the findings of secondary studies that are included in the current scoping review. One is meta-analysis, two are systematic reviews, and the other two are reviews. The publication year ranges from 2012 to 2022. One of the systematic reviews is without results because did not find any relevant intervention study related to preconception diet.
Quality of the Studies
The quality of the included studies was appraised (Supplemental Material 3) with an average quality score of 70±18%. Most of the observational studies were based on secondary data from a large cohort project. However, the quality assessment was made solely on how the authors’ reporting in the article. Three articles are considered poor, ten articles are fair, and 29 are good (marked in Table 4) [10–46]. Articles with poor quality were the two reviews and one RCT that did not provide enough information related to the study methods. Most of the studies that have fair quality did not mention the justification of the sample size, their study power and effect. The studies with fair quality also did not clearly explain whether the assessors were blinded to the exposure status, therefore, assumed as ‘No’ or ‘Not Clear’. The outcome in the fair studies was self-reported, and therefore considered to have a high risk of bias. Regardless of the rank of quality, most studies in use recall and only measured the exposure once.
Assessed Diet-related Exposure and Outcomes
We found 13 observations of dietary patterns and 16 observations of dietary quality (Figure 2B). The discussion of dietary pattern includes various known dietary patterns; Western diet; Prudent diet; Mediterranean diet; alternate Mediterranean diet; Nordic diet; and Diet to Stop Hypertension, and other defined dietary patterns; low caloric diet with controlled micronutrient; energy-dense poor-nutrient diet; and pre-defined healthy diet. Dietary quality includes observation for Healthy Eating Index, alternate Healthy Eating Index, and Prime Diet Quality Score. The mention of dietary pattern or quality score can be found in Table 1.
Maternal gestational diabetes mellitus (GDM) was the most discussed topic for the maternal outcome, with 28 observations for different diet-related exposures. Hypertension disorder in pregnancy (preeclampsia) and asthma were the second observed maternal outcome (n=4). Meanwhile, fetal and newborn anthropometry was the most observed child health outcome to different diet-related exposures (n=7) followed by preterm birth and neonatal morbidity (n=3).
DISCUSSION
The current scoping review observed that, to date, research related to pre-pregnancy diet was mostly conducted in the HIC population. The most observed area was North America (the United States and Canada) and the least observed based on the area was the African region. The dietary pattern and dietary quality were the two-most observed diet-related exposures. The most assessed outcome was GDM, hypertension disorder in pregnancy, and fetal and newborn anthropometry. FFQ was the most common dietary assessment tool used in research related to the pre-pregnancy diet.
Pregnancy is, most of the time, an unpredictable event. Even in the case of intended pregnancy, the starting point of early pregnancy can be unknown. Therefore, research related to pre-pregnancy diet often requires a long longitudinal observation from exposure to outcome. The time constraint explains the high number of studies that extracted secondary data from big longitudinal study. However, in studies using such method, the exposure and outcome assessments were sometimes cannot be implemented consistently.
The types of maternal morbidity are varying based on the region and types of economy. Hemorrhage, hypertension and preeclampsia, and sepsis were recognized as the leading cause of maternal mortality in high income countries. Hemorrhage, hypertension, dystocia, and sepsis in sub-Saharan Africa. Hypertension and hemorrhage in North Africa and Middle East. Hemorrhage, hypertension, anemia, and sepsis in Asia. Hypertension, hemorrhage, and infection in Latin America [10]. Hypertension, hemorrhage, and sepsis or infection are problems in all regions and economy, however, only three articles observed four dietary parameters to hypertensive disorder in pregnancy. Pre-pregnancy diet exposure to hemorrhage and infection were not found, but the nature of both etiologies was not directly related to diet and nutrition.
Anemia is one of the most prevalent maternal morbidities in Asia [52] and globally in LMICs and LICs with global prevalence of 42% in pregnant women [53]. Anemia is highly affected by nutrition [54]. Iron, folic acid, vitamin B12, and vitamin A deficiency, as well as protein energy malnutrition are among the contributing factors of nutritional anemia [53]. A population study in Indonesia shows that pre-pregnancy anemia increased the risk of child anemia in under-5 years old [55]. However, we did not identify any study of pre-pregnancy diet and anemia.
Malnourished mother’s nutrition is unlikely to suddenly improved during pregnancy due to even increasing nutritional demand. There are risk of insufficient weight gain and chronic energy and micronutrient deficiencies, that contribute to poor birth outcomes, neonatal mortality, and subsequent childhood malnutrition [56]. Newborn mortality incidence was reported the highest in sub-Saharan Africa, followed by Central and South Asia. Most neonatal deaths were related to preterm birth, childbirth-related complications, infections, and birth defects [57]. Malnutrition in children also contributes to under-5 death globally. Stunting and chronic protein-energy malnutrition are prevalent in Africa and Asia, while micronutrient deficiencies, mainly iron, vitamin A, iodine, and zinc, are prevalent in developing countries [56,58,59]. Referring to the burden of child mortality and morbidity, regions with high prevalence of child mortality still had the least number of available research in preconception diet and child health outcome. Micronutrient deficiencies in children are connected to maternal nutrition, such as iron storage in newborns that are affected by maternal iron [60]. In addition, evidence already supports the prevention of birth defects through adequate maternal nutrition, for example folate for neural tube defect prevention [61,62]. Maternal protein malnutrition also linked to hippocampal formation and neurobehavioral development [63,64]. But research related to preconception diet and child micronutrient deficiency, birth defect, and development were still scarce.
Although the evidence level was still weak, maternal nutrition is associated with intergenerational effects on NCDs risk in adult offspring [65]. Study in epigenetics suggests the role of nutrition in the early phase of life in the development of allergy, metabolic disorders risk, and cancer in the future [66–69]. However, further study is still required, including maternal pre-pregnancy diet’s role in the future NCDs risk.
Globally, there has been a shift in the diet not only in HICs but also in developing countries. The plant-based diet has been shifted to high-fat, energy-dense diet [70]. Adolescent diet practice is crucial that is likely to extend to adulthood and representative of pre-pregnancy diet [71]. Several research in adolescent dietary quality report the poor dietary habit in adolescent girls in LMICs [71,72]. Pre-pregnancy diet has not been attention in LMICs and LICs, although is recognized as a critical base for birth preparedness and health in the lower economy countries. Some experts agreed that pre-pregnancy care priority of urgency is high due to the remaining-high maternal and child mortality and morbidity in some regions. Research related to pre-pregnancy context also needs to consider different local circumstances and context [73]. The currently available studies were mostly in HICs, which may not be completely suitable for implementation in LMICs and LICs context.
The current study used systematic search method and additional hand search to obtain more articles that engaged with the topic. The quality of included articles was also assessed by its methodology. The approach also conducted by two assessors, which, lowers the risk of bias. However, some studies related to pre-pregnancy may be missed during the search process. Studies in other language from English and Bahasa were also not included and may cause bias in selection process. The current scoping review also only included primary original research and did not include review and grey literature.
CONCLUSION
Pre-pregnancy diet is a potential opportunity to prepare for the healthier next generation and stop intergenerational cycle of malnutrition. The current evidence related to pre-pregnancy diet is still limited. Future research is encouraged in LMICs and LICs contexts, as well as South-East Asia, Pacific, and African regions. Some maternal and child nutrition-related morbidity, such as anemia, micronutrient deficiencies, and birth defects have not much or at all discussed yet. More research on these aspects and the regional dietary context will benefit to fill in the gaps related to pre-pregnancy diet and maternal and child health.
SUPPLEMENTAL MATERIALS
Supplemental materials are available at https://doi.org/10.3961/jpmph.22.472.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
None.
ACKNOWLEDGEMENTS
None.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Wirawan F, Gayatri A. Data curation: Wirawan F, Yudhantari DGA, Gayatri A. Formal analysis: Wirawan F, Yudhantari DGA, Gayatri A. Funding acquisition: None. Methodology: Wirawan F, Yudhantari DGA, Gayatri A. Project administration: Wirawan F. Visualization: Wirawan F. Writing – original draft: Wirawan F, Yudhantari DGA, Gayatri A. Writing – review & editing: Wirawan F, Yudhantari DGA, Gayatri A.