Discrete-time Survival Analysis of Risk Factors for Early Menarche in Korean Schoolgirls
Article information
Abstract
Objectives
The aim of this study was to evaluate the effect of body weight status and sleep duration on the discrete-time hazard of menarche in Korean schoolgirls using multiple-point prospective panel data.
Methods
The study included 914 girls in the 2010 Korean Children and Youth Panel Study who were in the elementary first-grader panel from 2010 until 2016. We used a Gompertz regression model to estimate the effects of weight status based on age-specific and sex-specific body mass index (BMI) percentile and sleep duration on an early schoolchild’s conditional probability of menarche during a given time interval using general health condition and annual household income as covariates.
Results
Gompertz regression of time to menarche data collected from the Korean Children and Youth Panel Study 2010 suggested that being overweight or sleeping less than the recommended duration was related to an increased hazard of menarche compared to being average weight and sleeping 9 hours to 11 hours, by 1.63 times and 1.38 times, respectively, while other covariates were fixed. In contrast, being underweight was associated with a 66% lower discrete-time hazard of menarche.
Conclusions
Weight status based on BMI percentiles and sleep duration in the early school years affect the hazard of menarche.
INTRODUCTION
From menarche to menopause, women are exposed to endogenous estrogen. The timing and dose of life-long exposure to endogenous estrogen are known to affect various health conditions in women. The risks of cardiovascular disease, osteoporosis, and estrogen-dependent tumors such as breast cancer have been reported to be related to overall estrogen exposure and the onset of periodic exposure marked by menarche [1-3]. Early menarche is not only associated with later health outcomes but also unsafe sexual relations, substance use, adolescent depression, early termination of school education, and suicidal attempts [4-6]. Therefore, age at menarche (AAM), which provides information about the onset of periodic estrogen exposure and other related biological or psychological pubertal changes, is likely to be related to various adverse health outcomes later in the lifetime, especially for those who experience menarche at an early age [7].
Nonetheless, the reference range of AAM remains a controversial topic [8]. Various age intervals have been suggested based on studies of different ethnic groups and generations, but menarche before reaching 12-14 years is generally considered early menarche among researchers investigating the health risks of early menarche. Based on this definition, more attention needs to be paid to the global phenomenon of an increased early menarche prevalence [9-11]. The AAM of Korean girls is significantly declining at a faster rate than other populations [12].
Previous studies have suggested various individual risk factors related to early or late menarche. Despite considerable heterogeneity, longitudinal studies suggested that lower birth weight, higher body weight, and weight gain in infancy and early childhood are related to an increased risk of early menarche [13]. Factors indicating socioeconomic status at multiple levels, such as household income or socioeconomic deprivation of the school area, were found to be associated with earlier puberty. In contrast, strenuous exercise, being underweight, and long sleep duration have been reported to be related to delayed menarche. Knowing the factors that can affect the timing of menarche can help us develop interventions to modify the adverse outcomes associated with early menarche. In particular, research on the effects of weight status and sleep duration, which are relatively easy to modify, has important public health implications [14-18].
However, most previous studies modeling the relationship of sleep duration and body weight status with early menarche did not incorporate time-varying aspects of those variables. Children’s body mass index (BMI) and sleep duration are volatile variables that can change throughout growth. Previous studies dealing with BMI-based weight status included it as a single variable at specific critical points (e.g., BMI at the age of 8-9 years) or focused on differences in the BMI z-score within a defined interval. Therefore, an analysis that can account for the time-varying characteristics of these variables would be helpful [19,20].
Entrance to the formal schooling sector offers an excellent chance to monitor the phenomenon of early menarche and the effects of health-related behaviors on it. We hypothesized that BMI and sleep duration after school entrance are related to the hazard or conditional probability of menarche during observed time intervals.
METHODS
Data Source and Study Subjects
The 2010 Korean Children and Youth Panel Survey (KCYPS) was conducted by the National Youth Policy Institute (NYPI). The current study analyzed the KCYPS data on the elementary first-grader panel from 2010 to 2016. We considered incorporating the panels of elementary schoolers in fourth grade or children in the first year of middle school from the KCYPS 2010. However, considering the heterogeneity problem, we chose to focus on a single panel of the KCYPS 2010. To focus on the effects of time-varying variables such as BMI on the AAM, we decided on the youngest panel that allowed us to investigate conditions before the onset of menarche. According to the multiple-point prospective panel design of KCYPS, a probability sample (n=2342) representing all first-grade students in elementary schools in Korea was chosen and surveyed every year from 2010 until 2016. Stratified multi-stage cluster sampling was used. Seventy-eight schools were selected using the probability proportional to size sampling method. Then, for each school, a class was selected, and all students of that class and their guardians responded to the survey questionnaire. Individual longitudinal weights were calculated to account for the data representativeness and potential bias resulting from sample attrition and follow-up losses. Further study details are provided on the official website of the NYPI [21].
Since the AAM was investigated in wave 7, the study population was limited to the subgroup of female students who responded appropriately to the AAM question.
Because the first year of the panel study did not ask about the height and weight of students used to calculate BMI, we analyzed the data from the second year. The observed data were then transformed into a person-period data format of 4469 discrete time intervals of binary observations of 914 students until the event of interest (i.e., menarche) occurred or was censored to calculate the hazard.
Age at menarche
In wave 7 of the panel study, students were asked, “In what grade did your menarche happen?” The possible answers were: not yet, in third grade or earlier, fourth grade, fifth grade, sixth grade, or seventh grade. Those who responded “not yet” were considered right-censored, while those who answered “in third grade or earlier” were analyzed as having experienced menarche during third grade. Since data collection was conducted discretely every year, AAM was estimated to happen during the typical age interval of school grades in Korea. For example, those who responded that they experienced menarche during fifth grade were estimated to have experienced menarche between 11 years to 12 years of age. Interval censoring was considered in the main statistical analysis.
Exposures and covariates
All variables included in the model are time-varying variables collected from the multiple-point prospective panel data. To evaluate the effect of BMI, we classified the BMI status according to age-specific and sex-specific BMI percentiles according to the 2017 Korean National Growth Charts for children and adolescents: underweight (<5th percentile), normal (≥5th percentile and <85th percentile), overweight (≥85th percentile and <95th percentile), and obesity (≥95th percentile). The exact weight cut-off values for defined percentiles were based on the reference growth table developed by the Committee for the Development of Growth Standards for Korean Children and Adolescents, using the sixth month of the age interval of the measured grade [22,23]. To evaluate the effect of sleep duration, we calculated the exact duration in minutes based on weekday sleep onset time and wakeup time. Then, sleep duration was classified as short, adequate, or long based on the age group-specific recommendations from the National Sleep Foundation, which was 9 hours to 11 hours. [24]. Self-reported general health condition, which was answered with 1 of 4 levels (very good, good, poor, or very poor), was used as a covariate. This variable was dichotomized by merging the first and last 2 levels. Categorized annual household income was also included in the model as a covariate. Considering the right-skewed distribution of the variable, we created 10 intervals having equal length and then labeled the lowest interval as “low.” The second and the third lowest intervals were defined as “middle,” and the others were designated “high” [23].
Statistical Analysis
Discrete-time survival analysis methods can be used to extend the usability of survival analysis techniques, which usually assume continuous time measurement. In practice, data are often collected at discrete time intervals. For example, the current data (KCYPS 2010) are organized annually. Therefore, methods that do not require the assumption of a continuous measurement of time are needed. The fundamental measurement used to evaluate the risk of event occurrence in a discrete-time period is the discrete-time hazard, defined as the conditional probability that an individual will experience the target event in the time period, given that they did not experience it in earlier time periods.
This study modeled discrete hazards using a Gompertz model or a grouped proportional hazards model. Discrete hazard models can be embedded into the generalized linear model framework with an appropriate link function. There are multiple discrete hazard models, such as the logistic model, the probit model, the Gompertz model, or the Gumbel model. Among these models, the Gompertz model using a clog-log link is favorable because of its interpretability. The exponential term of a parameter estimate can be used directly to quantify the difference in the hazard value per unit difference in the predictor [25].
The current data were processed into the person-period format by assigning separate rows for each individual and each period when a person is observed until the event occurs or is censored, eventually turning them into multiple binary observations. Since discrete-time survival analysis describes the conditional probability of event occurrence, the records of the person-period data need only assume conditional independence. This means that multiple observations from the same individual do not harm the validity of estimates due to the violation of independence among observations [25].
The current model included weight status based on BMI, sleep duration, self-reported general health condition, and income as a categorical variable, as discussed earlier. “Exit” was additionally included in the model as a variable indicating discrete time intervals when data were observed. In this case, it was represented as the age at the time of the survey. We conducted analyses using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria), and the ‘lme4’ package was used.
Ethics Statement
The research protocol of the survey was reviewed and approved by the institutional review board associated with the National Institute of Youth Studies, Korea.
RESULTS
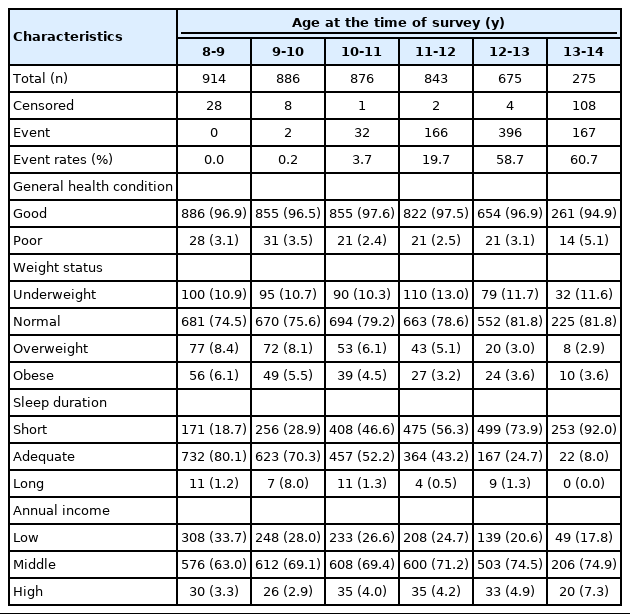
Table 1 presents the essential characteristics and distribution of covariates for each at-risk population at consecutive time intervals. The hazard increased as individuals reached the next time interval. It is noteworthy that the proportion of overweight participants decreased as they entered the next at-risk population without experiencing menarche in the earlier time intervals. Meanwhile, the proportion of underweight showed an increasing trend, though not monotonously. For sleep duration, the proportion of those who slept the recommended hours decreased as they reached the following time intervals.
Table 2 depicts the results of Gompertz regression of the discrete time-to-event data. The exponentials of coefficients of the variables are considered as relative hazards of a unit change of the variable while other variables are fixed. For example, the advancement of a 1-time interval (i.e., 1 year in this study) increased the conditional probability of an individual experiencing menarche in the present time interval by 2.84 times, given that the individual had not experienced menarche up to this point. Based on the statistically significant coefficients of our model, being overweight or sleeping less than the recommended duration was found to be related to an increased hazard of menarche compared to being normal weight and sleeping 9 hours to 11 hours, by 1.63 times and 1.38 times, respectively, while other variables were fixed. In contrast, being underweight decreased the hazard by 66%.
An additional analysis after merging the long and adequate sleep duration groups still showed a statistically significant coefficient, implying an increased hazard of sleeping less than the recommended hours (results not shown).
DISCUSSION
This study suggests that being overweight or sleeping less than 9 hours per day was related to an increase in the hazard of menarche. In comparison, being underweight decreased the hazard.
Our findings support previous studies about the relationship between AAM and body weight [13,23]. Many previous studies used previous BMI at fixed age intervals to study its effect on AAM. Behie and O’Donnell [23] reported that a higher BMI at age 8-9 increased the chance of earlier menarche. Kelly et al. [26] reported that BMI at 7 years increased the odds ratio of menarche before reaching 11 years. Adair [27] also reported that larger sum of skinfolds and higher BMI at 8 years were strongly associated with earlier menarche. The above studies included BMI as a continuous variable in their models. Yet, it is recommended to use weight status based on BMI-for-age percentile instead because children are growing and developing [22]. Seo et al. [8] reported trends in the prevalence of early menarche stratified by BMI status of Korean girls. The prevalence of early menarche was higher among the overweight and obese group than among the normal and the underweight group. However, in some years, the prevalence of early menarche was higher in the overweight group than in obese group. This result suggests that the risk of menarche may not increase linearly as BMI increases [8].
Several studies reported a relationship between sleep duration and menarche. Carr et al. [28] evaluated 44 children with treatment-resistant circadian rhythm sleep disorders and reported that 5 children with sleeping disorders developed precocious puberty, Implying that shorter sleep duration may trigger or accelerate menarche, as shown in the current study. Ku et al. [15] surveyed 411 North Korean refugees and reported that sleep duration was negatively correlated with AAM. This result conflicts with our result. However, Ku et al. [15] used a cross-sectional design, and the average age of participants was 31.3 years old. Therefore, they had to assume a consistent lifestyle to infer the impact of sleep duration on AAM. Disruption of temporality might have resulted in different outcomes.
Our results are biologically plausible. Excess adiposity or overweight status may accelerate pubertal changes through several mechanisms [13]. Adipose tissue produces various adipocytokines that may affect pubertal timing [29]. Leptin secretion due to energy sufficiency has also been suggested to accelerate this process [30]. The aromatase activity of fat tissue may also induce the peripheral conversion of androgens to estrogen [31]. There remains much to understand about the relationship between sleep duration, hormone secretion, and precocious puberty. The timing and duration of sleep can induce or interrupt hormone secretions, which may affect the timing of pubertal changes. Hormone secretion with sleep during puberty has been suggested as an essential biological index. Sleep is an important mediator of gonadotropin-releasing hormone secretion, and augmented luteinizing hormone secretion has been suggested to be synchronized with sleep cycles [32]. A decrease in plasma melatonin levels may also affect puberty onset in children. It has been suggested that a physiological decrease in melatonin plasma level activates the hypothalamus-pituitary-gland axis [33].
The study has some limitations, as follows. The measurement of the event in this survival analysis was based on self-report, which can result in recall bias [34]. Cooper et al. [35] evaluated the validity of AAM self-reported in middle age compared with that recorded in adolescence and showed moderate agreement (kappa=0.35, r=0.55).
Other known risk factors for early menarche include lower birth weight, weight gain in infancy and childhood, and a high intake of animal proteins and iron [13,36-39]. However, we did not consider these variables in our analysis because they were not included in the KCYPS 2010 data.
The potential misclassification of self-reported exposures is a study limitation. Since our study is based on a 7-year follow-up of elementary first-grade students, the accuracy of the answers given by students can be questioned. Due to the young age of the study population, for some variables, the parents were asked to respond instead of the students. For example, weight and height data were collected from parents before the students reached fourth grade. The potential bias in the data collection step can be a threat due to this heterogeneity.
Additionally, the sample size of our study was not very large. This small size resulted in some categorical variables with relatively few participants, which deteriorated the quality of coefficient estimation. In particular, the proportion of students with long sleep duration was very low.
Nonetheless, the current study has the following strengths. The current study used answers from the seventh year of follow-up to measure AAM. We can anticipate better validity than in previous studies due to the shorter timespan between the actual event and the recall time.
Furthermore, the temporal relationship between the exposures and the outcome of interest was well established due to the usage of multiple-point prospective data. By using longitudinal data collected every year, starting when no one had experienced menarche, we guaranteed that exposures preceded the outcome of interest.
Previous studies exploring possible risk factors of early menarche commonly employed logistic regression. To do so, the researchers had to devise reference values to define normal menarche operationally and define exposures as time-fixed variables of a particular critical timing or change of those variables in designated time intervals. Considering the lack of consensus regarding reference values for normal AAM, this can result in heterogeneous analyses [8,34,40]. Compared to logistic regression, discrete survival analysis using menarche as an event has an advantage for investigating the effects of timevarying exposures across time intervals without the need to devise different age criteria for normal AAM.
Our study showed that overweight status or sleeping less than 9 hours per day was related to an increased hazard of menarche, while being underweight reduced the hazard. Our study is the first to analyze the effects of these variables on the hazard of menarche in a single-panel study representing the nationwide Korean population.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
This work was partly supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea Ministry of Science and ICT (No. 2020-0-00121) and the National Research Foundation of Korea (NRF) grant funded by the Korea Ministry of Science and ICT (No. 2020R1A2C2101041).
AUTHOR CONTRIBUTIONS
Conceptualization: GIL YJ, Sung J. Data curation: Gil YJ, Park JH. Formal analysis: Gil YJ. Funding acquisition: Sung J. Methodology: Gil YJ, Sung J. Writing – original draft: Gil YJ. Writing – review & editing: Gil YJ, Sung J, Park JH.
ACKNOWLEDGEMENTS
None.