Projection of Cancer Incidence and Mortality From 2020 to 2035 in the Korean Population Aged 20 Years and Older
Article information
Abstract
Objectives:
This study aimed to identify the current patterns of cancer incidence and estimate the projected cancer incidence and mortality between 2020 and 2035 in Korea.
Methods:
Data on cancer incidence cases were extracted from the Korean Statistical Information Service from 2000 to 2017, and data on cancer-related deaths were extracted from the National Cancer Center from 2000 to 2018. Cancer cases and deaths were classified according to the International Classification of Diseases, 10th edition. For the current patterns of cancer incidence, age-standardized incidence rates (ASIRs) and age-standardized mortality rates were investigated using the 2000 mid-year estimated population aged over 20 years and older. A joinpoint regression model was used to determine the 2020 to 2035 trends in cancer.
Results:
Overall, cancer cases were predicted to increase from 265 299 in 2020 to 474 085 in 2035 (growth rate: 1.8%). The greatest increase in the ASIR was projected for prostate cancer among male (7.84 vs. 189.53 per 100 000 people) and breast cancer among female (34.17 vs. 238.45 per 100 000 people) from 2000 to 2035. Overall cancer deaths were projected to increase from 81 717 in 2020 to 95 845 in 2035 (average annual growth rate: 1.2%). Although most cancer mortality rates were projected to decrease, those of breast, pancreatic, and ovarian cancer among female were projected to increase until 2035.
Conclusions:
These up-to-date projections of cancer incidence and mortality in the Korean population may be a significant resource for implementing cancer-related regulations or developing cancer treatments.
INTRODUCTION
Controlling cancer requires monitoring its incidence and mortality rates. Due to aging, a growing population, and the changing prevalence and distribution of cancer risk factors, the burden of cancer incidence and mortality is growing rapidly worldwide [1]. Overall, cancer is the first or second leading cause of death before the age of 70 years in most countries, according to an estimate from the World Health Organization (WHO) in 2019 [2].
In 2015, more than 200 000 cancer cases were diagnosed and cancer was the leading cause of death in Korea [3]. In addition, according to a study that measured the burden of disease in Korea between 2008 and 2018, non-communicable diseases accounted for 86.8% of all disability-adjusted life years [4]. In 2016, a total of 229 180 cancer cases were newly diagnosed (120 068 in males and 109 112 in female) [3]. There were 78 194 cancer deaths (48 208 in male and 29 986 in females) [3]. According to a study predicting the 2020 burden of cancers in Koreans based on an analysis of historical trends, 243 263 cancer cases and 80 546 cancer deaths were expected, and the authors stated that cancer incidence and mortality were expected to increase over time [5].
The relationship between cancer mortality and incidence rates is complex. In terms of current trends, the mortality rate of cancer is expected to decrease given the increase in cancer diagnoses and treatment developments. However, the risk factors related to cancer incidence, such as aging, smoking, obesity, and Westernized lifestyle habits, have increased over time [6]. Changes in the population structure in Korea have also influenced the projections of cancer cases and deaths [7]. Therefore, comparisons of the age-standardized incidence and mortality rates for cancers considering the structure and distribution of the population each year are required [8]. Moreover, understanding the occurrence of cancer both enhances the awareness of its danger to people and is linked to policy issues that may lead to developments in cancer treatment and changes in insurance coverage. Thus, estimating the projected incidence and mortality rates of various cancers is critical to enable preparations for the near future, as well as for the more distant future in 2035.
In this study, we aimed to examine the long-term trends in the incidence and mortality rates of common, intermediate-frequency, and rare cancers in the Korean population from 2000 to 2035 using a joinpoint regression model.
METHODS
Data Sources and Selection
Data on cancer cases were extracted from the statistical database of the Korean Statistical Information Service from 2000 to 2017, and deaths were extracted from the National Cancer Center (NCC) from 2000 to 2018. Those data were split into the number of cases by 5-year age groups and sex. The cancer cases and deaths were classified according to the International Classification of Diseases, 10th edition. The cancer sites and codes that were included in this study were listed in Supplemental Material 1 and 2.
Statistical Analysis
The age-standardized incidence rates (ASIRs) and age-standardized mortality rates (ASMRs) were investigated using the 2000 mid-year estimated population. The age-specific rates for each cancer site were grouped according to the sex and 5-year age group (from 20 to 84 or 85+ years in incidence and from 20 to 79 or 80+ years in mortality). The age-standardized and age-specific crude rates for each year were calculated per 100 000 individuals.
First, to analyze the trends in cancer incidence and mortality, ASIRs from 2000 to 2017 and ASMRs from 2000 to 2018 were investigated in terms of annual percent changes (APCs) in joinpoint regression to detect the time points of changes in trends and determine the linear trends between these points [5]. More specifically, we used a joinpoint regression model (X=year, Y=ln(rate)) to estimate the average annual percent change (AAPC) of the ASIR and ASMR for each cancer [9]. The AAPC is a summary measure of the trend that uses a single number to describe the average APC over a period of multiple years. The analysis was performed using the Joinpoint program version 4.8.0.1, provided by the National Cancer Institute of the United States [10]. The Joinpoint regression software automatically selected the best-fitting trend pattern, using the permutation test to select the number of joinpoints. The confidence interval of each AAPC was also calculated based on a normal distribution [11]. Projections of cancer incidence and mortality in 2020, 2025, 2030, and 2035 were estimated by using the methods applied by Rahib and colleagues [12], which are shown in Supplemental Material 3.
The cancer incidence and mortality rates from 2020 to 2035 were calculated by dividing the number of cancer cases and deaths by the population values of the specific years according to sex, age, and cancer type. Furthermore, differences in cancer morbidity were estimated as rate ratios (incidence rate ratio [IRR] and mortality rate ratio) and rate differences (incidence rate difference and mortality rate difference).
Ethics Statement
The Institutional Review Board of Seoul National University Hospital approved this study (IRB approval No. 1911-188-18-084).
RESULTS
The standardized incidence rates of common, intermediate-frequency, and rare cancers in the Korean population aged 20 years and older are shown in Table 1. In terms of AAPC, thyroid cancer was projected to be the fastest-accelerating cancer (male: 15.0%, female: 10.9%), followed by kidney cancer (male: 4.6%, female: 4.8%). The largest change among male was projected to be in prostate cancer (AAPC, 8.10%; 95% CI, 7.05 to 9.17) and that among female was projected to be in breast cancer (AAPC, 5.87%; 95% CI, 5.00 to 6.74) (Table 1).

ASIRs (per 100 000 people) of common, intermediate-frequency, and rare cancers in the Korean population aged 20 years and older
Among adults over 20 years of age, using the annual median population in 2000 as the standard population, the trends of cancers between 2000 and 2035, the observed trends (2000-2017) and the projections (2020-2035), are shown in Figure 1. Among male, for common cancers, such as stomach, liver, lung, colorectal, and prostate cancer, the ASIR of prostate cancer was projected to increase significantly by 2035, which was an opposite trend to that of the other cancers (Figure 1A). The projected incidence rate of prostate cancer in 2035 was determined to be 189.53 per 100 000 people, corresponding to a 24.2-fold increase from the rate in 2000 (7.84 per 100 000 people) (Supplemental Material 4). In the analysis of the intermediate-frequency cancers, which include bladder, esophagus, pancreatic, and biliary tract cancer and non-Hodgkin lymphoma (NHL), the ASIR of NHL was projected to increase 1.68 times by 2035 (from 6.74 per 100 000 in 2000 to 11.34 per 100 000 in 2035), while that of esophageal cancer was projected to decrease sharply 0.37 times by 2035, which was 9.65 per 100 000 in 2000 to 3.61 per 100 000 in 2035 (Figure 1B). Among rare cancers, including cancers of the oral cavity and pharynx, kidney, larynx, leukemia, brain, and multiple myeloma, the ASIR of larynx cancer was projected to decrease, whereas kidney cancer was predicted to sharply increase over the next 15 years, to 26.39 per 100 000 people (Figure 1C). Among female, the projected ASIR of breast cancer was predicted to increase from 34.17 per 100 000 in 2000 to 238.45 per 100 000 in 2035 (Figure 1A). The ASIRs of intermediate-frequency and rare cancers are projected to increase steadily, except for bladder cancer, which showed an inverse trend (Figure 1B-C, Supplemental Material 4).

Projected cancer incidence rates in Korean male and female from 2020 to 2035. (A) Common cancers (B) intermediate cancers and (C) rare cancers. NHL, non-Hodgkin lymphoma.
Data on projected changes in mortality are presented in Table 2. In terms of the AAPC, larynx cancer was predicted to be the fastest decelerating cancer in terms of mortality (male: -9.6%, female: -12.9%). In contrast, the AAPC of multiple myeloma was 1.8% in male and 3.0% in female. Moreover, the ASMR of prostate cancer (AAPC, 0.83%; 95% CI, -0.33 to 2.00) among male was projected to increase from 3.29 per 100 000 in 2000 to 5.14 per 100 000 in 2035, with a mortality rate difFigure ference of 1.85 per 100 000. Among female, the ASMR of breast cancer (AAPC, 1.58%; 95% CI, 0.87 to 2.29) was predicted to increase by 4.10 per 100 000 from 2000 to 2035. Moreover, from 2000 to 2035, the ASMR was projected to increase by less than 2-fold for pancreatic cancer and ovarian cancer (mortality rate difference: 1.94 and 1.31, respectively) (Table 2, Supplemental Material 5).
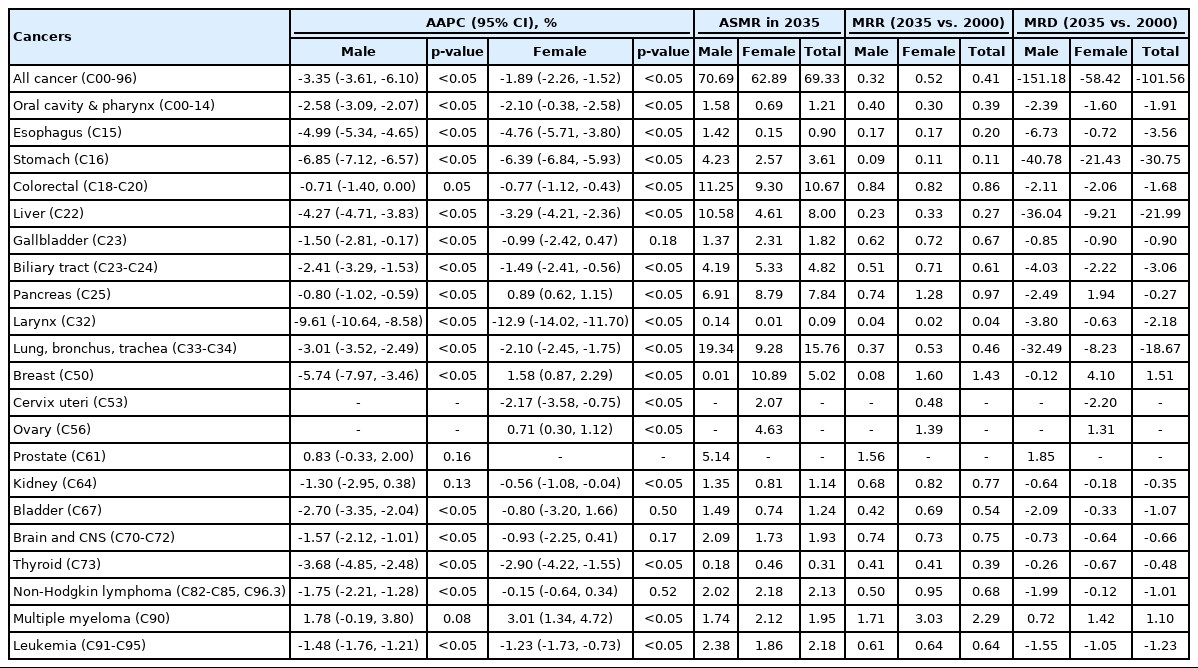
ASMRs (per 100 000 people) of common, intermediate-frequency, and rare cancers in the Korean population aged 20 years and older
The ASMRs from 2000 to 2035, which contain the observed trend (2000-2018) and projections (2020-2035), are depicted in Figure 2. Among, the ASMRs of the common cancers, including lung, stomach, liver, colorectal, and prostate cancers were predicted to decline dramatically, except for a projected slight decrease in mortality due to colorectal cancer (0.84 times lower in 2035 than in 2000) and a slight increase in prostate cancer (1.56 times higher in 2035 than 2000) (Figure 2A). Among the intermediate-frequency cancers, such as pancreatic, esophageal, bladder, and gallbladder cancer and NHL, esophageal cancer (the second leading cancer in 2020 and the expected fourth leading cancer in 2035) was predicted to show a rapid decrease in the ASMR (Figure 2B). Among the rare cancers, including leukemia, cancers of the oral cavity and pharynx, larynx, brain, and kidney, and multiple myeloma, larynx cancer was projected to have the lowest mortality rate in 2035 (Figure 2C). Among female, breast cancer, which is categorized as a common cancer, was projected to increase by 2035. Moreover, pancreatic and ovarian cancers, which are categorized as intermediate-frequency cancers, were precited to increase sharply by 2035 (Figure 2B).
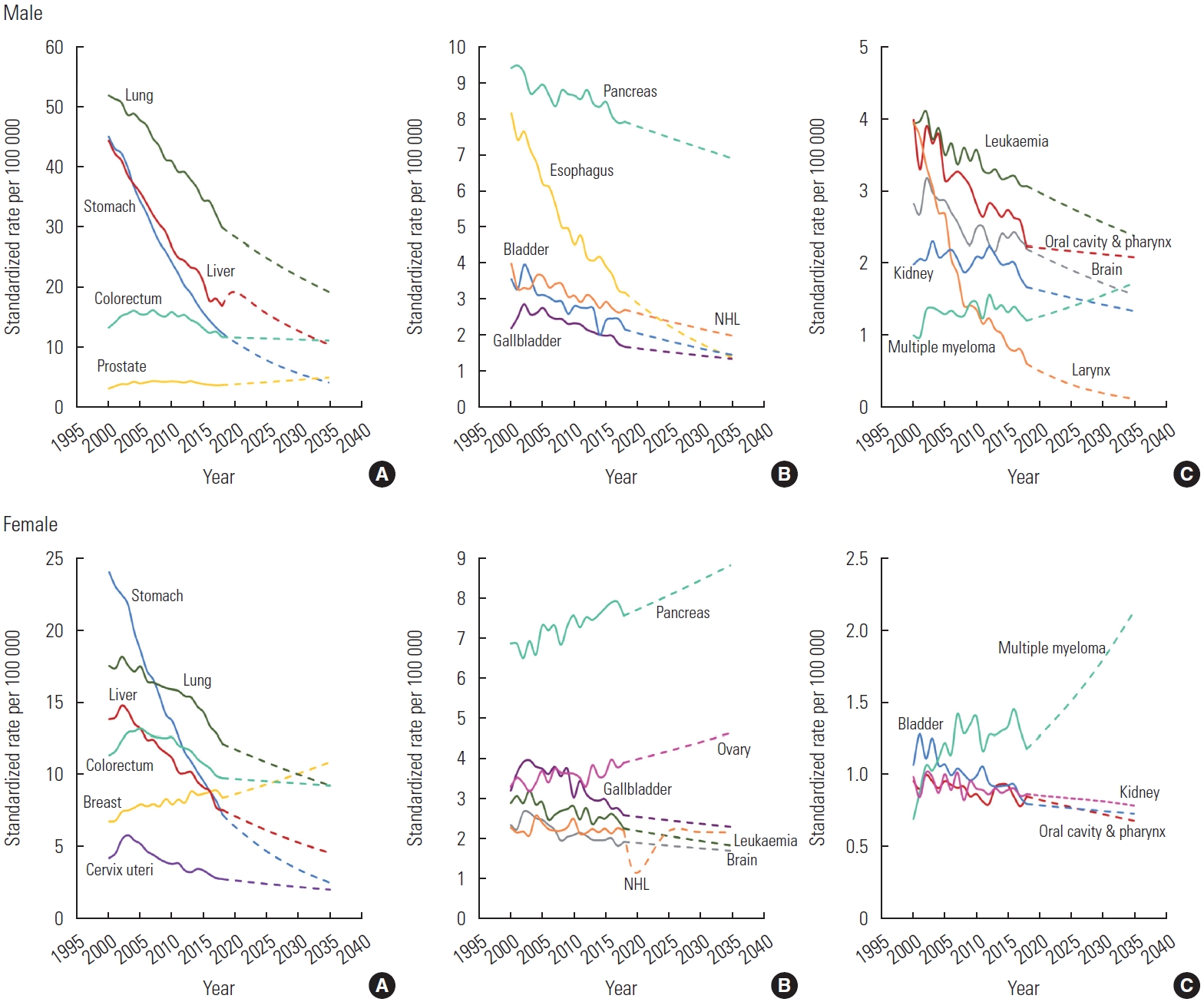
Projected cancer mortality rates in Korean male and female from 2020 to 2035. (A) Common cancers (B) intermediate cancers and (C) rare cancers. NHL, non-Hodgkin lymphoma.
The projected incidence and mortality rates from 2000 to 2035 with regard to the total cancers for male and female are shown in Supplemental Material 6. In both sexes, over the next 15 years, the crude and age-standardized incidence rates of total cancers were projected to increase, and the crude mortality rates and ASMRs of all cancers were projected to decrease. The ASMRs were predicted to decrease more sharply in male than in female.
The number of cancer cases and deaths from 2020 to 2035 in male, female, and both sexes are presented in Supplemental Material 1 and Supplemental Material 2. The number of total cancer cases was projected to increase from 265 299 in 2020 to 474 085 in 2035, an increase of 1.79%. In 2020, the leading cancers in both sexes were cancers of the thyroid, colorectal, stomach (especially non-cardia stomach cancer), lung, and liver (especially hepatocellular carcinoma). Furthermore, lung cancer was predicted to surpass non-cardia stomach cancer, resulting in it ranking third among the leading cancers by 2035. Breast cancer among female develops most frequently in postmenopausal female (over 50 years old), reaching 361.21 per 100 000 by 2035.
The projected number of total cancer deaths is expected to increase from 81 717 in 2020 to 95 845 in 2035, corresponding to an average annual increase of 1.17%, with lung cancer being the major cause of death from 2020 through 2035. The leading cancers in 2020 were those of the lung, liver, colorectal, stomach, and pancreas. In 2035, colorectal and pancreatic cancers were predicted to exceed the second and third most common malignancies, with biliary tract cancer being a common cancer.
DISCUSSION
In this study, we showed projections of cancer incidence and cancer mortality rates over the next 15 years (until 2035) and observed different trends for male and female. Prostate cancer among male and breast cancer among female were predicted to show the highest incidence rates of cancer. In addition, the overall mortality rates for both male and female were predicted to decline until 2035, except for breast, ovarian, and pancreatic cancer in female.
The ASIR of prostate cancer in male was projected to increase rapidly, whereas the ASMR was predicted to remain stable. Although the socioeconomic benefits of prostate-specific antigen (PSA) screening for the general population remain a matter of debate, compared to Western countries, PSA testing is not performed in regular check-ups in the Korean population. According to an analysis using Korean National Health Insurance Service data from 2008 to 2016, the prostate cancer-specific mortality rate of the population that did not receive PSA screening was almost twice as high as that among those who received PSA screening [13]. Moreover, prostate cancer incidence in male aged <70 years increased much faster than in those aged ≥70 years [14]. Therefore, PSA screening should be recommended.
With regard to breast cancer, the ASIR and ASMR in female were predicted to increase by 2035 compared to 2000. The increased incidence of breast cancer is due mainly to changes in reproduction, such as a decrease in the mean age at menarche, an increase in the mean age at onset of menopause, and a decrease in the fertility rate [15-19]. The mortality rate due to breast cancer was projected to increase more slowly than the incidence rate due to the increase in the overall survival rate as the result of the increased detection of early-stage breast cancer arising from national breast cancer screening for female over 40 years of age, with the greatest benefits among those aged 45-59, and the development of treatments [20,21]. For ovarian cancer, the ASMR was predicted to be approximately 1.4 times higher in 2035 than in 2000. Effective screening modalities or nationwide screening programs are needed to reduce the mortality rate of ovarian cancer, since ovarian cancer has the poorest survival rate because it is asymptomatic in the early stages and usually detected at an advanced stage [22].
In this study, thyroid cancer and renal cancer were projected to have high AAPCs for incidence in both sexes. The over-diagnosis of thyroid cancer has been a major issue in Korea [23,24]. Korea had 35 cases per 100 000 female in 1998-2002 and 260 cases per 100 000 female in 2008-2012, which was more than 8 times higher than the most up-to-date data based on the cancer registries in 26 countries [25]. With regard to renal cancer, the ASIR was projected to increase sharply from 2000 to 2035 in male, from 5.7 in 2000 to 26.4 per 100 000 in 2035 (corresponding to a difference in the incidence rate of approximately 20.7 per 100 000 male). Although the ASMR of renal cancer was predicted to decrease by -0.64, this would be a relatively insignificant change compared to the incidence rate. If an increase in cancer diagnosis occurs while there is no increase in cancer deaths, the occurrence of cancer is meaningless in the clinical context—or, in other words, the cancer is over-diagnosed [26]. Judging from the pattern of these results, it can be inferred that renal cancer may be over-diagnosed in the Korean population [26]. To prevent this, it is necessary to study the natural causes of renal cancer through additional research using cancer registration data managed by the Korea National Cancer Registry [27].
The Korean government founded the NCC in March 2000, legislated the Cancer Control Act in 2003, and in early 2006 planned a second-term cancer control plan for the following 10 years (2006-2015) [28]. The incidence and mortality of common cancers showed a decrease with the implementation of the National Cancer Screening program, while those of most cancers have also shown a decrease with the implementation of the Tobacco Quitline Program by the NCC and other agencies. According to the findings of this study, the incidence rates of cancers were predicted to decrease, while the survival rate was projected to increase due to treatment advances and earlier detection of cancer, which would concomitantly lead to a reduction in the mortality rates.
There are several limitations of the present study. First, there are many other methods of estimating the projection of cancer rather than the joinpoint regression model. For example, an age-period-cohort (APC) model reflects the cohort effect and shows the peak point of cancer cases or death in a certain age range [29], whereas it is impossible to infer the age range at which the number of cancer cases or deaths peaks by using joinpoint regression. However, an APC model has an identification problem due to the linear dependence of the 3 independent variables (cohort, age, and period). The prediction intervals of each cancer were not calculated in this paper, whereas these values can be estimated by using a Bayesian generalized APC power model [30,31]. Another limitation is that data on cases and deaths have already been updated; in particular, data on deaths in 2020 have been published. To verify whether the projections of this study were successful, the number of cases in 2019 and deaths in 2020 were compared as an observed/expected ratio (Supplemental Material 7 and Supplemental Material 8).
Some cancers showed dramatic trends, which might have been due to overfitting of the results. However, when the age-standardization year was changed to the 2010-mid-year population, the values of AAPCs and trends were similar. There was a difference in the structure of the population, wherein the population of those aged under 40 decreased and those aged over 40 increased in 2010 compared to 2000, for which reason the incidence based on the 2000 data appeared to have been higher. For example, the IRR of prostate cancer was 1.56 when using the 2000 population, but 1.31 when using the 2010 population (results were not shown). These effects might imply that there was no sudden difference in the trend projection, indicating that there was no significant difference according to the year chosen for the baseline analysis.
In an APC analysis, when observing only common cancers, the values of the goodness-of-fit differ substantially by sex and type of cancer. The incidence of thyroid cancer in both sexes and the incidence of prostate cancer and mortality of stomach cancer and lung cancer in male had goodness-of-fit values of more than 0.90. When using a Bayesian generalized APC power model, it appears that the incidence of cancer will increase over time. However, the prediction intervals are too wide compared to the observed year, and according to the 5-year age groups included in the analysis, it would be difficult to accurately analyze rare cancers or cases occurring at a young age (Supplemental Materials 9-16).
Overall, 474 085 new cancer cases and 95 845 cancer deaths are expected to occur in Korea in 2035. With the implementation of several systems for managing and preventing cancer, the ASIRs and ASMRs of cancers will continue to decline. The Tobacco Quitline Program of the NCC and improvements in screening are reflected in increased survival, decreased mortality, and a decreased incidence of cancers. Despite these positive findings, hematological tumors remain challenging.
Supplementary materials
Supplemental materials are available at https://doi.org/10. 3961/jpmph.22.128.
Notes
CONFLICT OF INTEREST
The authors have no conflicts of interest associated with the material presented in this paper.
FUNDING
This study was funded partly by the Korean Foundation for Cancer Research (Grant No. CB-2017-A-2) and a grant from the Seoul National University Hospital (2021).
ACKNOWLEDGEMENTS
None.
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Hong Y, Moon S, Choi J, Ko KP, Kim I, Lee JE, Park SK. Data curation: Hong Y, Lee S, Moon S. Formal analysis: Hong Y, Moon S, Lee S. Funding acquisition: Park SK. Methodology: Hong Y, Lee S, Moon S. Project administration: Ko KP, Kim I, Lee JE, Park SK. Visualization: Hong Y. Writing – original draft: Hong Y, Park SK. Writing – review & editing: Hong Y, Lee S, Moon S, Sung S, Lim W, Kim K, An S, Choi J, Ko KP, Kim I, Lee JE, Park SK.
