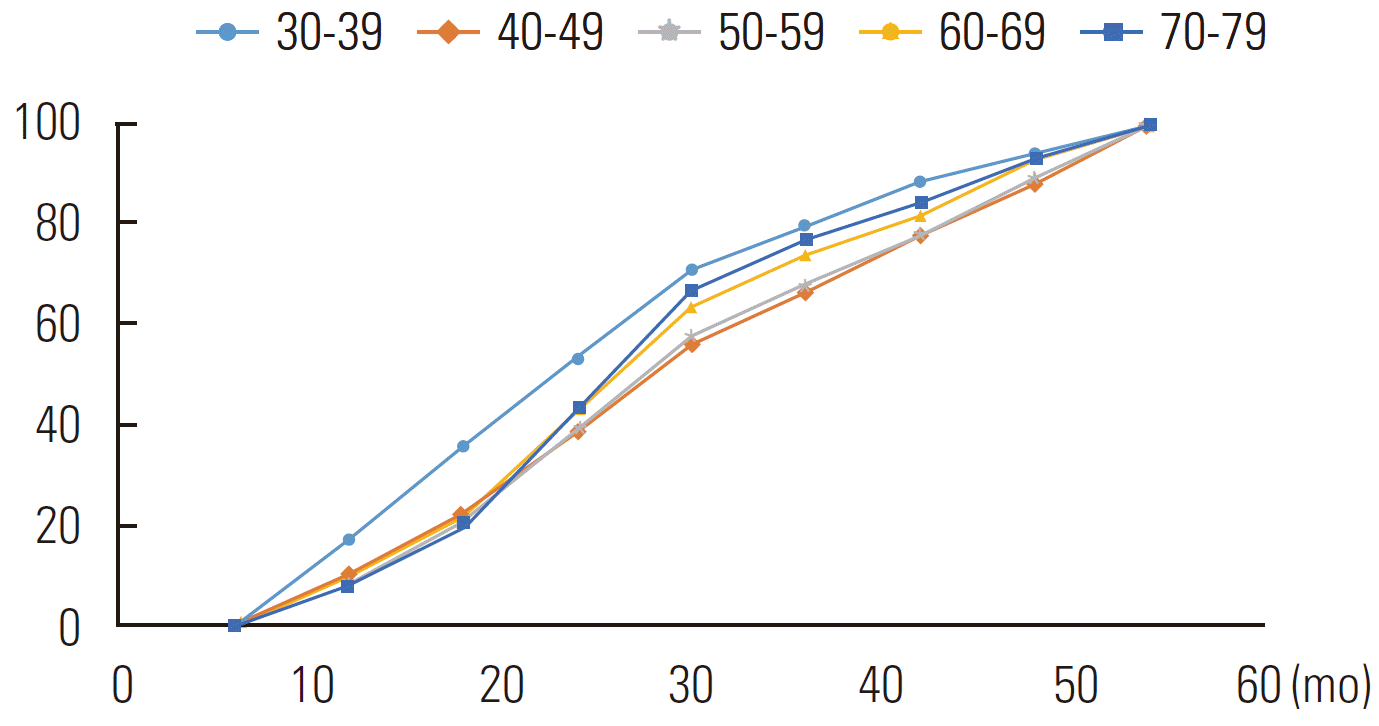Rates of Change to a Positive Result in Subsequent Screening Mammography in Korean Women: A Retrospective Observational Study
Article information
Abstract
Objectives:
This retrospective cohort study aimed at calculating some parameters of changes in the findings of the subsequent screening mammography (SSM) in female Korean volunteers.
Methods:
The study included screenees aged 30 to 79 years who underwent SSM voluntarily after testing negative in the baseline screenings performed between January 2007 and December 2011. A change to a positive result was defined as category 4 or 5 by using the American College of Radiology Breast Imaging Reporting and Data System. The proportion of results that had changed to positive (CP, %) was calculated by dividing the number of cases with results that were positive in the SSM by the total number of study participants. The rate of results that had changed to positive (CR, cases per 100 000 screenee-months) was calculated by dividing the number of cases with results that were positive in the SSM by the total number of months of the follow-up period.
Results:
The overall CP and CR in all age groups (n=77 908) were 2.26% and 93.94 cases per 100 000 screenee-months, respectively. The median CP interval in the subjects who had positive SSM results was 30 to 36 months, while that in the age group of 30 to 39 years was shorter.
Conclusions:
Different screening intervals should be considered among women aged between 30 and 59 years. In addition, a strategy for a screening program should be developed for the age group of 30 to 39 years, in particular.
INTRODUCTION
The breast is the leading site of cancer incidence worldwide [1], and the second leading site in Korean women [2]. While the pISSN 1975-8375 eISSN 2233-4521 global burden of breast cancer in women is substantial and increasing [3], 26 countries in the International Cancer Screening Network have operated a screening program developed on the basis of epidemiological characteristics in their countries [4]. Most programs focus on women aged 50 to 69 years with an interval of 2 years between screenings [5,6]. The National Cancer Center in Korea recommends biennial screening with mammography in women ≥40 years of age [7]. However, this recommendation was not made fully on the basis of epidemiological evidence of breast cancer in Korean women, although Korean women had an age-incidence curve markedly different from that of Western women [8].
To adapt the nationwide recommendation of cancer screening, any evidence related to the detection method, age groups covered, and recommended interval for modality should be obtained [9]. In particular, the age and interval between tests could be determined by estimating the mean sojourn time (MST) of the targeted cancer [10-12].
The MST could be defined as the mean time from the screening of a detectable size to that of a clinically detectable size [13]. If the date of subsequent screening showing positive findings was considered to be the date of clinical diagnosis, the duration between the date of baseline screening with negative findings and that of subsequent screening mammography (SSM) with positive findings could be considered the individually underestimated sojourn time in breast cancer [12]. Thus, the aim of the study was to calculate the proportions, rates, and periods of changing findings in the SSM among voluntary screenees to indirectly estimate the MST of breast cancer among Korean women.
METHODS
Study Population
The source population was the same as that in the study by Bae et al. [10] and Bae et al. [11]. In the screening programs provided by the Korea Medical Institute [14], full-field digital mammography is the primary modality for the initial and follow-up screening of breast cancer.
The selection criteria for constructing the baseline cohort from the source population were female Korean volunteers aged 30 to 79 years who underwent SSM after initial negative results during the first (baseline) screening between January 2007 and December 2011. The age at the time of screening was determined by the date of the baseline screening mammography starting in January 2007. Some SSMs conducted within less than 6 months of the previous test were treated the same as those in the previous test because the authors took into consideration that the tests would be performed by administrative issues such as conducting a cancelled screening, etc.
Definition of the Change to Mammographic Positivity
All screening mammograms were interpreted using the American College of Radiology Breast Imaging Reporting and Data System [15]. Among the five assessment categories, positive results were defined as category 4 (likely malignant) or 5 (malignant).
All screenees consented to undergoing screening mammography and the use of personal data for research. The study protocol was approved by the institutional review board of Jeju National University Hospital (file no. 2013-09-005).
Statistical Analysis
The subjects were classified into five age groups: 30 to 39, 40 to 49, 50 to 59, 60 to 69, and 75 to 79 years. The proportion of results that had changed to positive (CP, %) was calculated by dividing the number of cases with results that were positive in the SSM by the total number of screenees with negative results in the baseline screening. The follow-up periods stratified by age group were calculated as the number of months from the date of the first screening mammography to the date of a positive SSM result. If the last screening result was normal, the date of this test was considered to be the end of follow-up for that subject. From this follow-up information, the rate of change to a positive mammographic result (CR, cases per 100 000 screenee-months) was calculated by dividing the number of cases with results that were positive in the SSM by the total number of months of the follow-up period. The 95% confidence intervals (CIs) for CP and CR were calculated using the Poisson distribution. Lifetime method was used to estimate the cumulative CP at 6-month intervals. The log-rank test was conducted for testing the equality over age groups. A p-value of <0.05 was considered to be statistically significant in the two-sided tests. All statistical tests were performed using STATA version 12 (StataCorp, College Station, TX, USA).
RESULTS
Among the eligible population (n=188 022), 77 908 (41.44%) screenees underwent SSM (Table 1). The overall CP in all age groups was 2.66% among 77 908 subjects (Table 2). The youngest age group showed the highest CP, and CP decreased with an increase in age. The 95% CIs of CPs among the age groups of 30 to 69 years did not overlap. The overall CR in all age groups was 93.94 cases per 100 000 screenee-months (95% CI, 89.94 to 98.08). The CRs among all age groups had the same trend as the CPs (Table 3). The 95% CIs of CRs among the age groups of 30 to 59 years did not overlap.

Participating rates (%) of SSMs among the eligible population (n=188 022) who had negative results in the baseline mammography
Table 4 shows the cumulative CPs by age groups at intervals of 6 months. The median CP interval for the subjects with a positive SSM result was 30 to 36 months, while that for the group aged 30 to 39 years was shorter. In the log-rank test for equality over age groups, the p-value was <0.001. Figure 1 shows that the cumulative CPs in the age group of 30 to 39 years were the most different from those in the other age groups.

Cumulative proportion (%) of results that changed to positive results, as obtained using the life table method
DISCUSSION
Although, to the best of our knowledge, there has not been a previous study evaluating the parameters of changing SSM results of Korean screenees, the overall CR implies that 1 case of 12 772 screenees per year had a positive SSM result in the age group of 30 to 79 years. On the basis of cumulative CPs, one-half of the screenees with a positive SSM result had a changed result during 30 to 36 months, without an overlap with the 95% CIs for the age group of 30 to 59 years. However, these findings may be indirect evidence for determining the interval of SSM in healthy Korean women because these parameters did not suggest the MST directly, but estimated it indirectly [12].
Some statistical methods to estimate the MST by using Monte Carlo-Markov models have been reported [16]. However, they require the knowledge of the nationwide mammographic sensitivity by age group among Korean women [13]. The direct estimation of MST could not be determined, because nationwide cancer-related information could not be obtained easily owing to legal barriers in Korea [10-12]
Nevertheless, the age group of 30 to 39 years showed higher CP, higher CR, and earlier changes in the cumulative CP than the other age groups. These findings are in agreement with the findings of a previous study that reported breast cancer with a shorter sojourn time (ST) and more aggressive progression among younger women [6]. These findings suggest that in Korean women, the strategy of screening for breast cancer for the age group of 30 to 39 years should be different from that used for the other age groups [8,17-19]. In other words, these findings are the rationale for the suggestion of shorter screening intervals in the younger age group [20].
Several limitations should be considered for a valid interpretation of our results. The first is the assumption in calculating the individual ST. It is certain that the date of SSM with positive findings was not the same as that of clinical diagnosis, because some cases with positive SSM findings would be diagnosed as breast cancer later. However, the date of SSM with positive findings preceded that of the final diagnosis; thus, the estimated individual ST in this study was likely to be an underestimate of the actual ST. The second limitation is the self-selection bias. Because the study subjects were voluntary attendees of screening, they did not represent Korean women on the whole. Although these screenees may have been more interested in cancer prevention, the findings obtained from them could still be used as evidence supporting the recommendation of a national cancerscreening program for Korean women because they received results only after undergoing SSM. The third limitation is the inability to evaluate SSM performance at other institute. On the basis of a retrospective cohort study design, the authors calculated the CR by using screenee-months. The last limitation is the degree of accuracy in reading the screening mammograms. But our study used readings from a single institution, the conclusions can be expected to have been more reliable than they would have been from multiple institutions, and the level of validity of the readings at our institution would not be different from the nationwide value.
In conclusion, different screening intervals should be considered for the age groups of 30 to 39, 40 to 49, and 50 to 59 years. In addition, a strategy for a screening program should be suggested for the age group of 30 to 39 years, in particular. Because the incidence rate of breast cancer is increasing, the suggested screening guidelines for the early detection of breast cancer in Korean women should be adapted regularly on the basis of the related evidence obtained from Korean women [8,17-19,21]. Thus, an additional study for the direct estimation of MST in breast cancer using Monte Carlo-Markov models should be performed after obtaining nationwide information on the sensitivity by age group to correctly interpret the results of this study [12].
ACKNOWLEDGEMENTS
This study was supported by the 2013 project funded by the Korean Foundation for Cancer Research, Seoul, Korea (no. 2013-2). The authors thank Jee-Eun Kim from Jeju National University, Jeju, Korea, who helped in the construction of the Korea Medical Institute databases.
Notes
Conflict of Interest
The authors have no conflicts of interest with the material presented in this paper.