Physical Activity in Adolescence Has a Positive Effect on Bone Mineral Density in Young Men
Article information
Abstract
Objectives
Little is yet known about the determinants of bone mineral density (BMD) in young adults. Thus, in this study, we aimed to determine the factors that have an impact on BMD in young men.
Methods
Questionnaires were sent out to 111 male medical students. Information on age, socio-economic status, medical history, lifestyle, physical activity during adolescence, school club participation, current physical activity, and dietary intake were collected by the survey. Height, weight, percent body fat and muscle mass were estimated by bioelectrical impedance, and BMD was obtained using calcaneal quantitative ultrasound. Using the Poisson regression model, prevalence ratios (PRs) were used to estimate the degree of association between risk factors and osteopenia.
Results
The height and current physical activity showed a correlation to the Osteoporosis Index. Among the categorized variables, past physical activity during adolescence (p=0.002) showed a positive effect on the bone mineral content. In the multivariate model, past physical activity (≥1 time/wk) had a protective effect on osteopenia (PR, 0.37; 95% confidence interval [CI], 0.18 to 0.75) and present physical activity (1000 metabolic equivalent of task-min/wk) decreased the risk of osteopenia (PR, 0.64; 95% CI, 0.44 to 0.91).
Conclusions
Past physical activity during adolescence is as important as physical activity in the present for BMD in young men.
INTRODUCTION
Osteoporosis is a systematic condition of the bones that decreases the bone mineral density (BMD) and leads to an increased risk of fracture [1]. The prevalence of osteoporosis is expected to increase along with the growing senior population [2]. According to a report from the World Health Organization (WHO), the number of hip fracture incidents resulting from osteoporosis was 1.7 million in 1993 and is expected to rise to 6.3 million by 2050 [3]. In Korea alone, the number of hip fractures in women increased from 250.9/100 000 persons in 2001 to 262.8/100 000 in 2004, a 4.7% increase [4]. Extensive research has demonstrated that low BMD is the major cause of osteoporosis in the elderly [5,6]. On the other hand, other studies have shown that having a BMD above a certain level could decrease the risk of osteoporosis-related bone fractures [6].
Efforts have been made to identify the population that has low bone mass with more than one risk factor for fracture [7]. BMD differs according to a person's gender, weight, and body mass index (BMI) [8]. Physical activity is one of the important factors in bone health. Previous studies have shown that physical activity has a positive effect on bone mass during adolescence [9]. Significant bone loss due to lack of exercise was observed in patients that were put on prolonged bed rest [10]. BMD is known to be influenced by nutritional factors. Obesity, through several mechanisms, was found to affect bone metabolism. High intake of fat significantly decreased the rate of bone formation [11]. Calcium supplementation may alter the bone content. Studies have shown that the intake of calcium has positive effects on bone health in the young and elderly [12]. Moreover, the intake of calcium with vitamin D in combination was found to reduce the rates of non-vertebral fracture among elderly women [13]. A high prevalence of vitamin D deficiency was found to be linked with low BMD [14]. Vitamin K supplementation played a role in increasing the BMD of the lumbar spine [15].
Lifestyle habits also have an effect on bone mass. Years of cigarette smoking in elderly men was found to be associated with bone status [16]. Alcoholism is also known to be a risk factor for osteoporotic fractures and low BMD. Heavy drinkers were found to have a lower BMD compared to people who consume 0.5 to 1.0 drink per day [17]. Previous studies have suggested that caffeine consumption has a negative effect on BMD [18] though some other studies have failed to find such an effect [19].
Most studies have focused on the bone mass of postmenopausal women or the elderly. However, considering that adult BMD is dependent on the peak bone mass (PBM), which is reached in the adolescent or young adult period [20,21], evaluating the factors affecting the BMD in these periods would be worthwhile. Because the age of PBM formation and age-related changes in BMD differ by sex [21], the factors affecting bone formation and resorption would also differ a bit by sex.
In our study we aimed to assess the possible risk factors related to BMD in young men. To determine which factors were associated with the BMD of university students, we included their past and current physical activity, school sports club activity, diet, and lifestyle as a part of the survey.
METHODS
Subjects
A total of 129 students of Chung-Ang University, College/School of Medicine were recruited from January to March 2011. Only the male students were included in the study. The purpose and the method of the study were explained to the participants prior to the collection of the data. The study was approved by the institutional review board of the Chung-Ang University College of Medicine (2010-12-3) and informed consent was obtained from all of the subjects. 6 subjects were excluded due to inaccurate measurement and 12 were removed because they did not fully fill out the survey. In the end, 111 subjects were enrolled in the study.
General Information and Lifestyle
General information such as age, socio-economic status, and medical history and lifestyle (smoking habit, alcohol use, caffeine consumption, and use of calcium or vitamin D supplements) were collected by a self-administered questionnaire.
Anthropometry and Bone Ultrasound
The IOI-353 (Jawon Medical, Gyeongsan, Korea) placed in the department lab was used to measure the height and weight of the students. The height and weight were measured to 0.1 cm and 0.1 kg, respectively. The participants were asked to remove their outerwear when their weight was measured. Using the same machine, the fat rate and muscle mass were estimated by the bioelectrical impedance. The BMD of the participants was obtained using the calcaneal quantitative ultrasound Osteo Pro (BM Tech, Seongnam, Korea). The machine was calibrated using the standard material included in the kit. Based on the BMD results, the T-score, Z-score, and Osteoporotic Index were recorded after calculation with the device software. The degree of BMD was classified into one of three categories: normal (T-score ≥-1), osteopenia (-2.5< T-score <-1.0) and osteoporosis (T-score ≤-2.5) [22].
Physical Activity
Past physical activity was denoted as ≤1 time/mo, 2-3 times/mo, 1-3 times/wk, 4-6 times/wk, ≥7 times/wk of exercise during the high school period. This was then re-categorized into 2 groups: greater than or equal to 1 time a week, and less than 1 time a week. The current physical activity was calculated using the Global Physical Activity Questionnaire developed by the WHO, based on the metabolic equivalent of task (MET) values [23]. In addition, whether the student was a member of a school club that mainly involved physical activity was recorded along with the amount of time spent per month.
Current Dietary Intake
The dietary intake data was mainly focused on the amount of calcium and vitamin D consumption. The food-frequency questionnaire consisted of 23 items on foods that were good sources of calcium or vitamin D. The information to produce the list was obtained from the USDA National Nutrient Database for standard reference, release 24 [24]. Participants were asked how often they consumed each type of food. The amount of calcium and vitamin D intake was calculated using the same reference.
Statistical Analysis
Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). Spearman's rank correlation, the t-test, and ANOVA were performed according to the type of the variable. The age, weight, height, body fat mass, body mass index, current physical activity, calcium, and vitamin D intake were put in correlation with the Osteoporotic Index (OI). For further evaluation, risk factors such as the BMI, socio-economic status, past physical activity, current physical activity, school club activity, calcium intake, and vitamin D intake were categorized into several groups. The chi-squared test and Fisher's exact test were used to analyze the categorized factors. Multivariate Poisson regression was used with robust variance [25] to estimate the PR and 95% confidence interval (CI). Covariates found to be associated with osteopenia assuming a threshold level of significance of 0.20 were included in the initial multivariate Poisson regression model. To control for potential confounders, we also included variables for socio-economic status, smoking, daily calcium intake, daily vitamin D intake, and caffeine consumption.
RESULTS
We identified 111 subjects. Their characteristics, OI, T-score, Z-score, and statistical data from the questionnaires are shown in Table 1. The mean OI was 53.2, and the average T-score was -0.11. Among the 111 subjects, the BMI values of 54 subjects (48.6%) were less than 23, while 30 subjects (27.0%) had a BMI between 23 and 25. 27 subjects (24.3%) had a BMI greater than 25. 65.8% of the subjects had regularly exercised greater than or equal to 1 time a week during the high school period. 34.2% of the subjects had exercised less than 1 time a week. The average amount of physical activity was 1703 MET-minutes a week. The average intake of calcium was 658.8 mg per day and average intake of vitamin D was 130.2 IU per day. The average intake of caffeine was 86.6 mg per day.
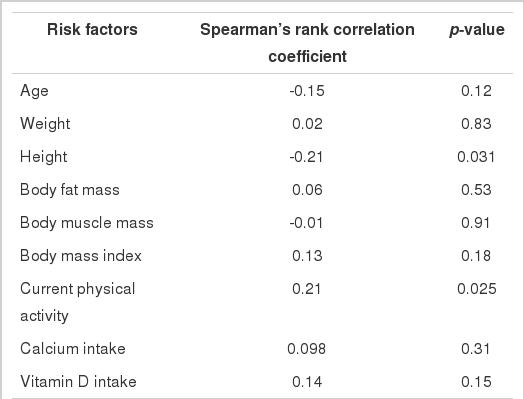
Risk factors that were correlated with the OI are shown in Table 2. Height (p=0.031) turned out to be negatively correlated with the OI, while current physical activity (p=0.025) showed a positive correlation with the OI.
Table 3 lists the risk factors that were categorized and put in the chi-squared test. Our results show that past physical activity (p=0.002) was associated with osteopenia, which is defined as a T-score <-1.0. Past physical activity (p=0.026) showed a significant positive association with the OI, which also supported its influence on the bone health of young men (data not shown).
Table 4 shows unadjusted and adjusted PR estimates and corresponding 95% CIs for the association between the risk factors (current physical activity and past physical activity) and osteopenia. Past physical activity (PR, 0.41; 95% CI, 0.20 to 0.84) and current physical activity (PR, 0.71; 95% CI, 0.53 to 0.96) were independently associated with frequency of osteopenia. These findings were sustained even after adjusting for factors such as socio-economic status, daily calcium intake, daily vitamin D intake, and caffeine consumption.
DISCUSSION
Previous studies have been focused on the BMD of postmenopausal women, which is less meaningful in terms of prevention because the principal cause of bone loss is closely related to hormonal effects. Our results showed that aging, low BMI, low physical activity, and low past physical activity were independently associated with the presence of osteopenia.
Although the age range was narrow (19 to 34 years old), we observed that age had a positive effect on osteopenia. The BMD gradually increases during the first two decades of life before reaching a plateau, and then decreases. The age at which PBM is reached is still controversial. In the Caucasian population, PBM is reported to be reached at the end of the second decade to the early part of the third decade [26]. However, recent studies performed in Korean or Japanese populations have reported that the PBM was reached around 20 years old [21,27].
Age-related bone loss is known to be caused by the increase of bone resorption and decrease of bone formation. This is due to a shift from osteoblastogenesis to predominant adipogenesis in the bone marrow [28].
In our study, BMI showed a significant negative relationship with osteopenia. Anthropometric parameters such as height, weight, and BMI were known to affect the BMD [28-32]. Tsukahara et al. [29] showed that the most important risk factor of low BMD during adolescence was low body weight because increased BMI adds a mechanical load to the skeleton and creates a positive effect on bone density. The presence of excessive fat tissue increases the calcium absorption, decreases the sensitivity to parathyroid hormone, and increases transformation of androstenedione to estrone [28].
Our results showed that current and past physical activity affected the prevalence of osteopenia independently. Furthermore, past physical activity during high school significantly reduced the PR even more than current physical activity. It has been determined that physical activity has positive effects on BMD across the age spectrum [33]. Weight-bearing physical activity augments the bone mass compared to non-weight-bearing sports. Exercise in early puberty greatly enhances bone strength as compared to that at a later age [33,34]. Recent evidence suggests that physical activity in childhood is one of the most powerful preventive strategies in fighting against osteoporosis [35]. In the present study, only physical activity ≥1 time a week (more than 30 min) reduced the risk of osteopenia.
Although present physical activity had a positive effect on BMD, being a member of a school athletic club and regularly participating in the club did not. This result provides us with the valuable information that in terms of bone health, one should focus on regular exercise rather than joining a school athletic club. Due to the heavy loads of learning and long hours of studying, it is difficult for medical students to maintain the amount of exercise they need to prevent bone loss [36]. This study has shown that physical activity in adolescence is as important as that of the present.
Recently, physical exercise class in high school seems to be undervalued. The physical exercise classes are converted to a different lecture or are just a class in name only but used as a study hall. According to our results, this could prevent adolescents from gaining bone health because physical activity in adolescence affects the BMD of young men. Our results suggest that policies should be modified towards reinforcing physical education in high school classes.
Dietary factors such as calcium, vitamin D, and caffeine consumption are well known risk factors of osteoporosis. Calcium intake during adolescence is reported to have a significantly positive effect on BMD [37]. However, some studies based on an adolescent and young adult population did not find a statistically significant relationship between calcium or vitamin D intake and BMD [38,39]. In this study, these factors did not affect the BMD or the prevalence of osteopenia. When we analyzed the data, we used the calculated amount of calcium and vitamin D intake from the food frequency questionnaire. Because these dietary data may not represent the actual status of serum calcium or vitamin D, further studies will be needed.
The effect of smoking on BMD is controversial. In some studies, both current and former smokers were at greater risk of having low BMD compared to nonsmokers [30]. Our results did not show a positive correlation between smoking status and BMD. Probably a low smoking rate (18.9%) and short period of smoking in our study population could have affected these results.
In our study, the Osteo Pro, a portable calcaneal quantitative ultrasound was used to evaluate the BMD. Although this type of device has been used in several previous studies [31,32], and was proven to be as sensitive as the dual energy X-ray absorptiometry (DEXA) scan [40], the standard tool used for assessing BMD in most of the health care facilities is the DEXA scan. We chose to use the Osteo Pro to avoid the risk of irradiation. In addition, we needed a portable device, as some of the measurements were taken outdoors.
This study was limited to the students currently enrolled in medical college/school. In order to discuss the risk factors associated with the BMD of young men, we will need to widen the selection of the participants. There are also some recall biases because we collected the data such as past physical activity via questionnaire survey. To elucidate the importance of past physical activity on maintaining bone health, we need further research using a prospective design that can collect more precise information about physical activity and diet history during the school-aged years.
In conclusion, we showed that past physical activity during adolescence is as important as the physical activity in the present in affecting the BMD in young men. We hope that this report will inspire interest in preserving the bone health of young men, leading to the understanding of the importance of regular exercise in adolescence.
Notes
The authors have no conflicts of interest with the material presented in this paper.