Effects of Smoking on Menopausal Age: Results From the Korea National Health and Nutrition Examination Survey, 2007 to 2012
Article information
Abstract
Objectives:
Decreased fertility and impaired health owing to early menopause are significant health issues. Smoking is a modifiable health-related behavior that influences menopausal age. We investigated the effects of smoking-associated characteristics on menopausal age in Korean women.
Methods:
This study used data from the Korea National Health and Nutrition Examination Survey from 2007 to 2012. Menopausal age in relation to smoking was analyzed as a Kaplan-Meier survival curve for 11 510 women (aged 30 to 65 years). The risk of entering menopause and experiencing early menopause (before age 48) related to smoking were assessed using a Cox proportional hazards model.
Results:
The menopausal age among smokers was 0.75 years lower than that among non-smokers (p<0.001). The results of the Cox proportional hazards model showed pre-correction and post-correction risk ratios for entering menopause related to smoking of 1.26 (95% confidence interval [CI], 1.09 to 1.46) and 1.27 (95% CI, 1.10 to 1.47), respectively, and pre-correction and post-correction risk ratios for experiencing early menopause related to smoking of 1.36 (95% CI, 1.03 to 1.80) and 1.40 (95% CI, 1.05 to 1.85), respectively.
Conclusions:
Smokers reached menopause earlier than non-smokers, and their risk for experiencing early menopause was higher.
INTRODUCTION
Menopause is an inevitable physical change experienced by women during the aging process. As the production of female hormones decreases and ovulation stops alongside the aging of the ovaries, regular menstruation disappears. Although menopause itself is not a pathological condition, menopausal transition is accompanied by negative physical changes. With the increase in life expectancy, the proportion of postmenopausal life also increases. Thus, the impact of menopause on women’s health has become more significant from both clinical and health perspectives [1-3].
According to previous studies, reaching menopause earlier increases the risk of overall mortality by approximately 2% per year, and also increases the risk of cardiovascular disease, type 2 diabetes, and osteoporosis. Menopause is also associated with dyslipidemia [3], hypertension [4], and ischemic stroke [5]. Furthermore, in women experiencing early menopause, the menopausal syndrome negatively affects mental health (leading for example to anxiety and depression) [6], and along with a dysfunctional sex life and loss of the reproductive period; in turn, it causes a significant decrease in the women’s quality of life [2].
Natural menopause is affected by genetic [7], environmental [8], and sociodemographic factors [9]. The average age of menopause of Korean women is known to be 49.4 years, and women from Lebanon, Singapore, Greece, Morocco, Mexico, Taiwan, and Turkey have an average age at menopause of 47 to 50 years [10]. All of these are lower than the age at menopause of women in the US of 50 to 51 years [9]. It has also been reported that various factors such as education level [9,11], occupation [9], use of oral contraceptives [9,12], age at menarche [13], obesity [8], smoking [9,11,14-16], alcohol consumption [8], and physical activity [8] affect the age of menopause.
Among these factors, smoking, a modifiable health-related behavior, has been studied as an influential cause of accelerating the onset of natural menopause. Bernhard [17] first presented a negative correlation between smoking and age at menopause among American women in 1949. After this early study, this correlation has been reported using various methodologies [9,11,14-16,18,19]. In most studies, the age of menopause among smokers was significantly earlier than among non-smokers. Other studies have reported an association of the age at menopause with smoking period, smoking quantity, and the age at starting smoking [11,20].
The percentage of women smokers in Korea was 5.8% in 2012, which was the lowest rate among the Organization for Economic Cooperation and Development countries [21]. Most previous studies on the relationship between smoking and menopausal age have been conducted in western countries with a relatively high smoking rate in women [22]. Thus, evidence on whether smoking would be related to menopausal age in the same manner in a region with a low smoking rate among women is insufficient. One meta-analysis showed heterogeneity in the relationship between menopausal age and smoking even among countries in the same continent [23], there might be some differences of susceptibility among different ethnic groups [24].
Therefore, the purpose of this study was to analyze the effects of smoking, an important factor in women’s health, on menopausal age using Korea National Health and Nutrition Examination Survey (KNHANES) data collected from a nationally representative sample population. The percentage of women smokers in recent years has increased, especially among women in their 20s, and was expected to be higher considering the fact that it is underreported due to social and cultural characteristics [25]. By evaluating the impact of smoking on women’s health using representative data for Korean women, this study was expected to help establish future smoking cessation policies for women.
METHODS
Data Source and Study Subjects
This study used raw data from the 4th and 5th editions of the KNHANES, conducted from 2007 to 2012 by the Korea Centers for Disease Control and Prevention. Eligible participants are shown in Figure 1. Of a total of 13 707 women were aged 30 to 64 years, 13 605 were selected as study subjects after excluding 102 women who did not respond to the question regarding menstruation status or who responded with “do not know.” Among these, 849 women who responded that artificial menopause was the cause of their menopause and 1246 who responded that that they did not know their age at menopause or with missing answers to the age at menopause and smoking were excluded, leaving 11 510 women for the final analysis (Figure 1).
Variables
This study used the variables of sociodemographic characteristics, reproductive characteristics, smoking characteristics, and the survey year. The sociodemographic variables included age, education level, and employment status. Education level was categorized into three groups: middle school graduate or lower, high school graduate, and college graduate or higher. Employment status was defined as ‘yes’ if one respondent earned income through employment for more than 1 hour in the past week, worked for more than 18 hours as an unpaid family worker, or was on temporary leave.
The variables related to smoking characteristics included smoking status, smoking quantity, and age at starting smoking. Regarding ‘smoking status’, ‘smokers’ were defined as those who currently smoked, ‘past smokers’ as those who smoked in the past but did not currently smoke, and ‘non-smokers’ as those who never smoked. Women who had smoked less than 100 cigarettes during their lifetime were also considered as ‘non-smokers’. To reflect the smoking status at the time of menopause, smokers who started smoking after menopause were reclassified as ‘non-smokers’. For the variable ‘average daily smoking quantity’, 2 groups were created: ‘less than 10 cigarettes per day’ and ‘more than 10 cigarettes per day’. For ‘age at first starting smoking’, subjects were also subdivided into 2 groups: ‘age < 20 years’ and ‘age ≥ 20 years’, based on the self-reported age in the questionnaire.
The causal variables related to reproductive characteristics were oral contraceptive use, estrogen replacement therapy use, pregnancy history, and age at first menstruation, whereas the outcome variables were menstruation status and age of menopause. Pregnancy history was divided into 2 groups: ‘0 to 3 times’ and ‘4 or more times’. For the variable ‘age at first menstruation’, the average age of the respondents was used as the reference, and women were again divided into 2 groups: ‘early (age≤13 years)’ and ‘late (age>13 years)’.
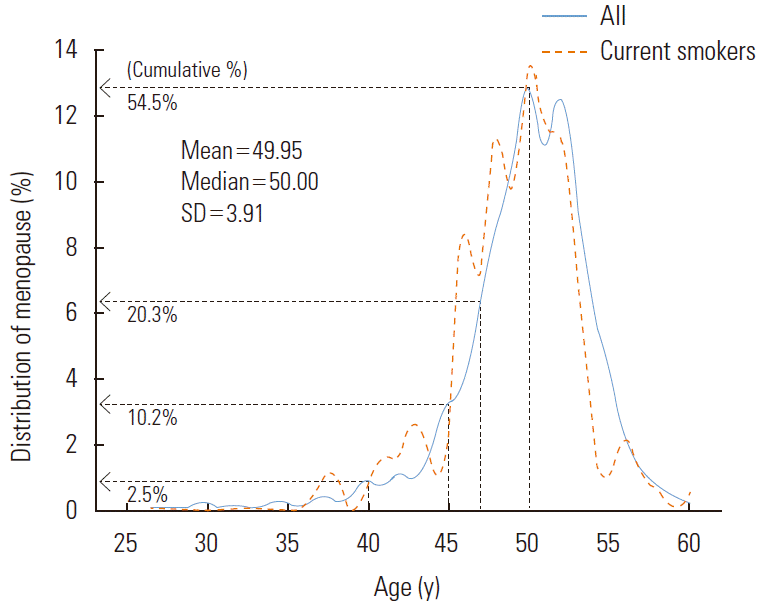
Natural menopause is defined as 12 months of continued amenorrhea without any clear pathological or physiological causes. Thus, the variable ‘current menstruation status’ was divided into ‘pre-menopause’, ‘natural menopause’, ‘pregnant or lactating’, ‘artificial menopause’, and ‘do not know’. Women who responded ‘do not know’ or ‘artificial menopause’ were excluded. For women in menopause, their self-reported age at menopause was used as the outcome variable, and the group was further divided into ‘menopause before age 48’ (which would be considered relatively early) and ‘menopause at age 48 or older’. Studies on early menopause have been conducted using a variety of criteria, with premature ovarian failure generally defined as occurring in women younger than 40 years old. The disorder has been known to occur in 1% of women. For early menopause, varying criteria have been used, including ages of 40 or 45 years. In this study, we calculated the risk for early menopause based on the lower 20% age tier, using an age of younger than 48 years as the clinical cut-off (Figure 2).
Statistical Analysis
We analyzed the distribution of variables for the study population (n=11 510) and menopaused group (n=4004), and calculated the average age at menopause and significance probability. To minimize the influence of the subject’s current age on the analysis, age was controlled by analysis of covariance.
A Kaplan-Meier survival analysis and log-rank test was performed to assess the correlation between smoking and age at menopause. Then, the risk, confidence interval, and significance probability for menopausal status and early menopause were calculated based on smoking status and characteristics using the Cox proportional hazards model. For characteristics related to smoking for more than 3 groups, such as smoking quantity and start of smoking, a trend analysis was additionally performed by using a linear trend chi-square test.
PASW version 19.0 (IBM Corp., Armonk, NY, USA). was used for data analysis, and statistical significance was set at p<0.05.
RESULTS
The general characteristics of the study population were shown in Table 1. The average age of 11 510 subjects at the time of the survey was 45.25 years (standard deviation [SD], 9.72), and the number of postmenopausal women was 4004 (36.0%).
Regarding smoking status, 6.6% of women were current smokers, 2.2% smoked in the past but did not currently smoke (data not shown), and 91.1% were non-smokers. In comparison to the average age at menopause of 49.99 years for non-smokers and past smokers, current smokers had a younger age at menopause of 49.24 years. The average daily smoking quantity was 8.21 cigarettes for current and past smokers, and the proportions of those who smoked less than 10 cigarettes and those who smoked more than 10 cigarettes were 81.6% and 18.4%, respectively. The average age at starting smoking was 24.5 years, and 24.9% of women started smoking before the age of 20, whereas 75.1% started at or after the age of 20.
The mean age at menopause adjusted for age showed significant differences by survey year, education level, age at menarche, use of contraceptives or estrogen replacement therapy, and smoking status. The menopausal age was earlier in the group with the lower education level, while no statistically significant difference was observed by employment status. Among the characteristics related to reproductive characteristics, the menopausal age was significantly earlier among women who had experienced relatively early menarche, or had used contraceptives or estrogen replacement therapy.
The distribution of the menopausal age is presented in Figure 2. The average age at menopause was 49.95 years (SD, 3.91), and the median age was 50.00 years. The distribution for the age at menopause ranged from 15 to 60 years, and 5 women reported their age at menopause as less than 30 years (including 2 women with ages at menopause at 15 and 20 years, respectively). Among the menopausal women, the number of women allocated to the early menopause group (experienced menopause before the age of 48) was 812 (20.3%).
The relationship between smoking status and the menopausal age was assessed by a Kaplan-Meier survival analysis and is presented in Figure 3. The average age at menopause of the smokers was significantly lower than that of the non-smokers (log-rank statistical test, p<0.001). However, the difference between groups according to the quantity of smoking or age at starting smoking was not statistically significant (log-rank statistical test, p=0.191, 0.208, respectively).
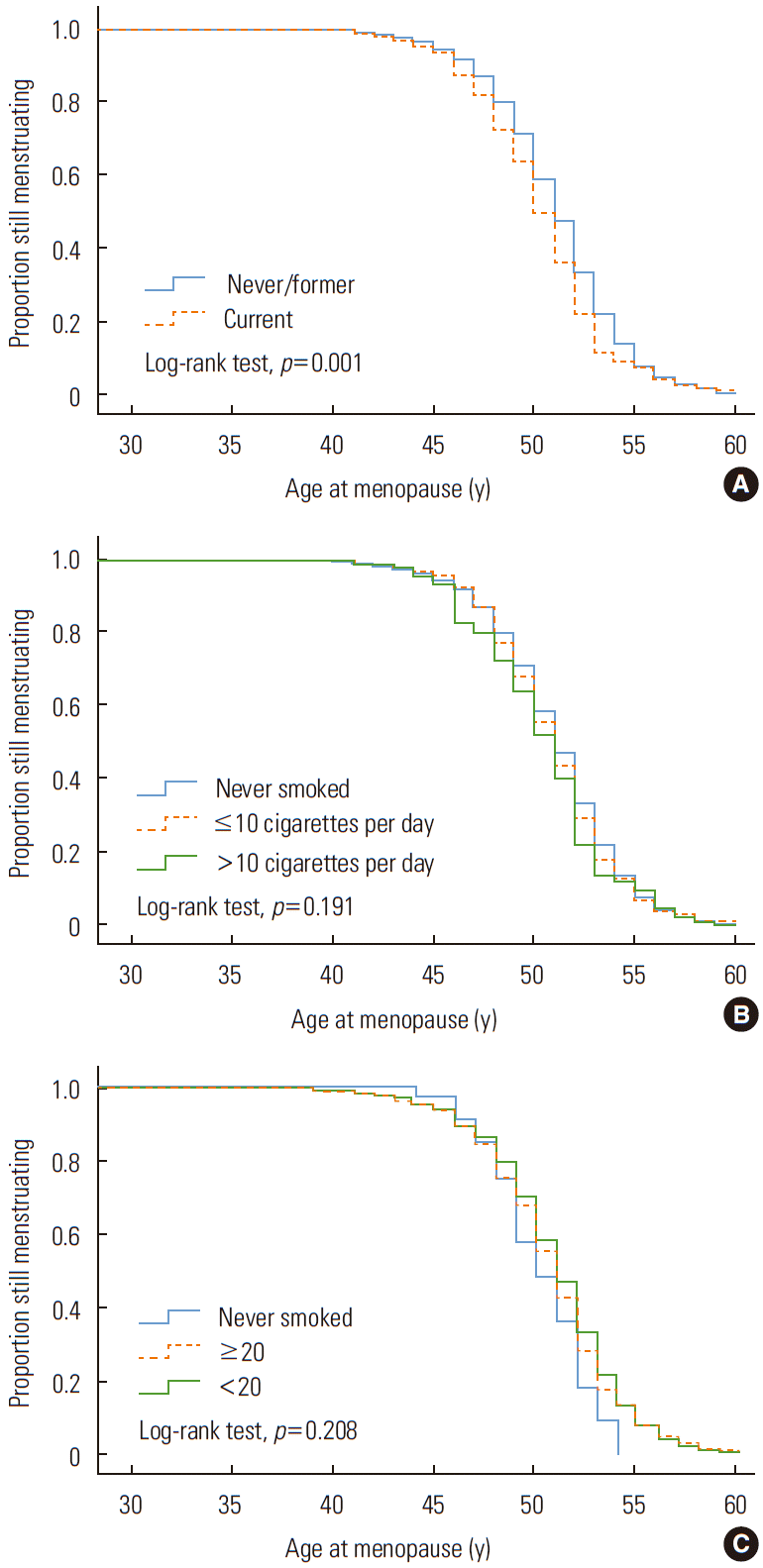
Kaplan–Meier survival curve of age of menopause stratified by (A) smoking status, (B) daily smoking quantity, and (C) age at starting smoking.
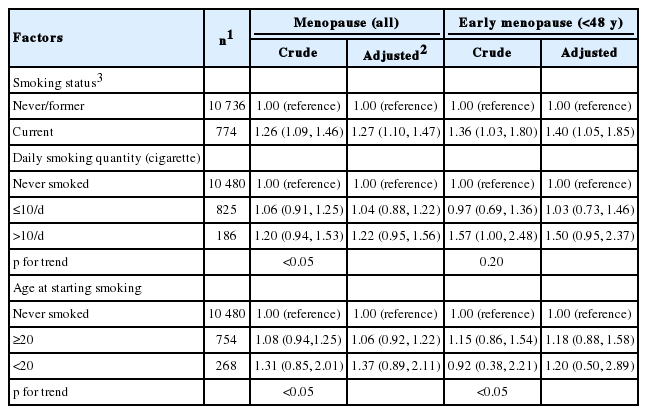
The risks associated with menopause and early menopause (before the age of 48) related to smoking characteristics such as smoking status, smoking quantity, and age at first starting smoking were calculated by Cox proportional hazards model and were presented in Table 2. We corrected for the variables that previously showed a significant difference for the age at menopause (survey year, education level, age at menarche, use of contraceptives, use of estrogen replacement; Table 1). The pre-correction and post-correction risk ratios for menopause were 1.26 (95% confidence interval [CI], 1.09 to 1.46) and 1.27 (95% CI, 1.10 to 1.47), respectively, and the pre-correction and post-correction risk ratios for early menopause (below age 48) were 1.36 (95% CI, 1.03 to 1.80) and 1.40 (95% CI, 1.05 to 1.85), respectively, with both showing statistical significance (p<0.05).
Non-smokers were compared to those who smoked less than 10 cigarettes and those who smoked 10 cigarettes or more. The results for the pre-correction and post-correction risk ratios for menopause were insignificant, but in the linear chi-square test, a pattern of increasing risk ratio was seen for those who smoked less than 10 cigarettes and those who smoked 10 or more cigarettes (p for trend <0.05).
Although the risk ratios associated with menopause or early menopause related to the age at starting smoking was not significant compared to non-smokers, the linear chi-square test indicated a significantly increasing trend for the risk for both menopause and early menopause compared to non-smokers when the age at starting smoking was lower (p for trend <0.05).
DISCUSSION
In this multiple cross-sectional study integrating 6 years of KNHANES data, the effects of smoking on the menopausal age in women were investigated.
The average age at menopause of women in this study was 49.95 years, which is similar to that reported in previous studies [10,20]. Socioeconomic status such as higher educational level and less financial strain has been shown to be correlated with an older age at menopause [9,10]. Consistent with prior studies, our results showed that higher educational level was significantly associated with higher menopausal age after adjusting for the age.
Regarding the reproductive factors, the age at menopause was higher in those who had experienced their first menstruation at a later age, which was consistent with the results of other studies [13,26]. However, while the age at menopause was lower among women who had taken contraceptives in our study, another study showed that contraceptive use was associated with a higher age at menopause [9,12]. Although it was proposed that contraceptive use and pregnancy postpone the menopausal age by inhibiting follicle depletion [20], a recent prospective study did not show any significant association [11]. Confounding variables such as lifestyle, and socioeconomic factors could have biased our results. Additionally, it should be considered that the presence of menstruation would not be a reliable marker of ovarian follicular reserve. Further investigation should be needed to demonstrate the valid protective mechanism.
Furthermore, estrogen replacement therapy is used to alleviate menopausal symptoms resulting from early menopause and it can artificially prolong menstruation or induce periodic uterine bleeding. So, resulting from the limitations of observational studies, the reported menopausal age of the treatment group might be approximated to the mean than the true natural menopausal age.
The percentage of current smokers among the women in this study was 6.7%. The age at menopause of current smokers was significantly lower than that of non-smokers, and past smokers showed a slightly higher age at menopause than non-smokers; however, this difference was not statistically significant (n=256; the age at menopause of past smokers=52.1±0.7 [age adjusted mean±standard error]). This was consistent with the majority of other reports, which showed that the age at menopause among current smokers was significantly lower than that among non-smokers while past smokers either showed a very modest increase or decrease in age at menopause or no significant difference [9,11,20]. One study suggested that the effects of smoking on the ovary might not be irreversible [27], which was contrary to many existing theories about permanent toxic effect from smoking. But, at the same time, the study suggested the possibility that the results could have been due to measurement error from smoking factors or menopausal age instead [20,28]. Furthermore, it could be possible that her health improvement efforts including quitting smoking were more effective than negative effect from past smoking in limited duration and amount, paradoxically leading to a higher menopausal age.
A variety of hypotheses on how smoking affects menopause have been proposed, and one hypothesis posited that polycyclic hydrocarbons lowered the in vivo estrogen level by acting on ovarian germ and follicle cells [29]. Moreover, in vitro experiments have shown that alkaloid compounds in cigarettes such as nicotine or cotinine inhibited the conversion of androstenedione to estrogen [30].
The risks associated with menopause and early menopause related to the smoking quantity and time of exposure showed no significant results by Cox proportional hazards model. However, in the linear trend analysis, we found a pattern of higher risks related to a higher smoking quantity for menopausal age and an earlier age at starting smoking for both menopause and early menopause. In other large-scale study, partial associations of the menopausal age with daily smoking quantity, accumulative smoking amount, and duration of smoking were observed [11,16]. It might be possible that our study only demonstrated partial associations due to the limited sample size.
The smoking rate among Korean women is much lower than that among women from other countries. However, according to Jung-Choi et al. [25] who compared the cotinine-measured smoking rate (13.9%) to the self-reported smoking rate (5.9%) among Korean women, the self-reported smoking rate seemed to be underreported. When interpreting the results of our study, it must be considered that observed association of smoking and menopause risk could be negatively biased from under-reporting of smoking status.
Including described above, our study has several limitations. It used a retrospective survey method, errors could have occurred when estimating the temporal cause and effect relations. Because the information has been collected by self-reporting method, bias from missing values and recall error could be involved. In particular, the menopausal process occurs over a certain period of time and usually is not clinically diagnosed, making it difficult to accurately recall and report the exact time point of menopause. And, the survey included women from wide age groups, and there might be a possibility of survival bias. Some potential confounders could not be assessed, including secondhand smoke.
Additionally, women experiencing artificial menopause were excluded from the sample selection process. This decision was based on the assumption that if the natural ages at menopause were observed among women with artificial menopause, the age distribution would have shown the same age distribution as for the women experiencing natural menopause. However, if there was any influence of smoking on artificial menopause, the possibility of a bias from underestimating the relationship between smoking and early menopause resulting from artificial menopause acting as a competitive variable for early menopause could not be excluded.
Despite several limitations, our study was the first study in Korea reporting the effects of smoking on age of menopause in women. Its strength might lie in the fact that the sample was highly representative as the data used for analysis were derived from KNHANES. In addition, the effects of smoking on menopause were analyzed for the entire population of reproductive age by using survival analysis. As a result, Korean women who continued to smoke had a younger age at menopause as well as an increased risk of experiencing early menopause than women who were non-smokers. In women, a younger age at menopause could lead to a higher morbidity and mortality for various diseases [31]; thus, the results of our study had important significance for public health. Furthermore, by identifying the effects of smoking on women’s health, the results suggested that a goal-oriented smoking cessation campaign for women might be necessary. In the future, a prospective study examining the cause and effect of various smoking characteristics on the age at menopause along with the effects of smoking cessation should be conducted.
Notes
Conflict of Interest
The authors have no conflicts of interest with the material presented in this paper.