Serum Uric Acid Level and the Incidence of Metabolic Syndrome in Middle-aged Korean Men: A 5-Year Follow-up Study
Article information
Abstract
Objectives:
Elevated serum uric acid (UA) has been known to be associated with the prevalence of metabolic syndrome (MetS). However, no prospective studies have examined whether serum UA levels are actually associated with the development of MetS. We performed a prospective study to evaluate the longitudinal effects of baseline serum UA levels on the development of MetS.
Methods:
A MetS-free cohort of 14 906 healthy Korean men, who participated in a medical check-up program in 2005, was followed until 2010. MetS was defined according to the Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention. Cox proportional hazards models were performed.
Results:
During 52 466.1 person-years of follow-up, 2428 incident cases of MetS developed between 2006 and 2010. After adjusting for multiple covariates, the hazard ratios (95% confidence intervals) for incident MetS for the second, the third, and the fourth quartile to the first quartile of serum UA levels were 1.09 (0.92-1.29), 1.22 (1.04-1.44), and 1.48 (1.26-1.73), respectively (p for trend <0.001). These associations were also significant in the clinically relevant subgroup analyses.
Conclusions:
Elevated serum UA levels were independently associated with future development of MetS in Korean men during the 5-year follow-up period.
INTRODUCTION
The prevalence of metabolic syndrome (MetS) is increasing worldwide, and in the Republic of Korea (hereafter, Korea) has been substantial since the late 1990s [1]. MetS is a clustering of metabolic disturbances such as abdominal obesity, high blood pressure (BP), increased blood glucose level, and dyslipidemia, all of which increases the risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (DM) [2]. In 2012, heart disease and DM were the second and the fifth leading causes of death in Korea, respectively. Several biomarkers, such as alanine aminotransferase (ALT), white blood cell count, and uric acid (UA), have been reported to play an important role in the development of MetS in Korean adults [3]. Moreover, associations with apolipoprotein B, serum ferritin, urine albumin, and non-alcoholic fatty liver disease have been observed in our prior studies.
UA is the metabolic end-product of purine metabolism in humans, and hyperuricemia has been linked with hypertension, insulin resistance, DM, and atherosclerosis [4]. Although it is still unclear if UA is a biomarker for concurrent lifestyle habits or is involved in the causal pathways leading to metabolic disturbances, UA is a potential target for preventing decreases in insulin sensitivity and other adverse prognosis associated with MetS [5]. In Korea, three cohort studies showed that elevated serum UA level is a strong and independent risk factor of MetS incidence [6-8], but two of these cohorts had a short follow-up period (1-3 years), small cohort (1437-4779 men), and lacked data on serum insulin level, the homeostasis model assessment of insulin resistance (HOMA-IR), and renal function in their data analyses. The remaining cohort study observed the incidence of MetS in a relatively large sample (10 802 men) for up to seven years, but only specific risk factors for MetS were assessed among the 2496 incident cases of hyperuricemia.
Therefore, we performed a follow-up study on a large number of initially MetS-free, healthy middle-aged Korean men (n=14 906), to evaluate the longitudinal effects of baseline serum UA levels on the development of MetS.
METHODS
Study Design
A prospective cohort study was conducted to investigate the association between serum UA levels and the development of MetS. Study participants consisted of Korean men undergoing a medical health check-up program at the health promotion center of a hospital in Seoul, Korea. The purpose of the medical health check-up program is to promote the health of the employees and to enhance the early detection of existing diseases. All employees participate in either an annual or biennial health check-up, as required by Korea’s Industrial Safety and Health Law. Most of the study population is comprised of employees and their family members who work at various companies within Korea. Their employers pay for the majority of the costs of the medical examinations. We took advantage of this opportunity to conduct a prospective study.
Participants
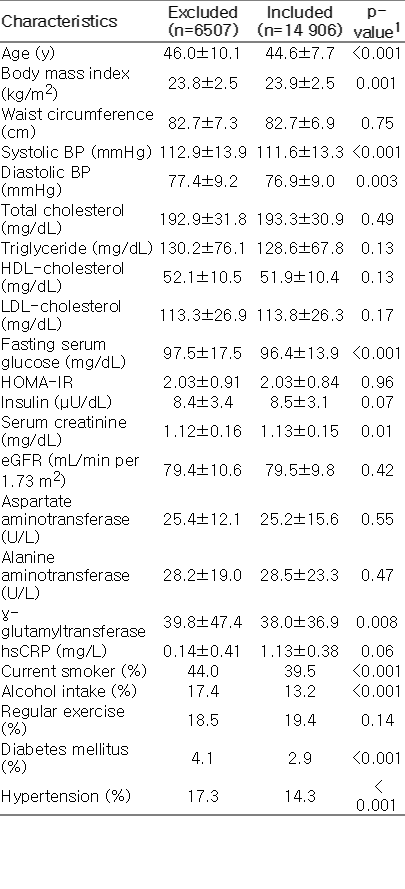
In total, 28 426 men who had been examined for all of the components of MetS during a medical check-up in 2005 were eligible for participation in this study. Among the 28 426 participants, 7013 were excluded. Subjects were excluded because they had already been diagnosed at baseline (the initial examination) with MetS (n=5353), had a history of CVD (n=226), had a history of malignancy (n=149), or were already taking lipid-lowering agents (n=2107), therefore leaving 21 413 eligible participants. We further excluded 6507 participants who did not attend any follow-up visits between 2006 and 2010. After exclusion, 14 906 participants were enrolled in the final analysis and observed for the development of MetS. The total follow-up period was 52 466.1 person-years, and the mean±standard deviation follow-up period was 3.52±1.49 person-years. Ethical approval for the study protocol and analysis of the data were obtained from the institutional review board of the hospital.
Clinical and Laboratory Measurements
Data were collected via the participant’s medical history, a physical examination, a questionnaire, anthropometric measurements, and laboratory measurements. The medical and drug prescription history were assessed by the examining physicians. All participants were asked to respond to a health-related behavior questionnaire, which included questions on alcohol consumption, smoking, and exercise. Alcohol intake was recorded as the frequency of alcohol consumption per week and the typical amount that was consumed per day (≥20 g/d). Participants who reported that they smoked at the time of the questionnaire were classified as current smokers. In addition, participants were asked how frequently they engaged in physical activities each week that lasted long enough to produce perspiration, such as jogging, bicycling, and swimming (≥1 time/wk). DM was defined as a fasting serum glucose level ≥126 mg/dL or the current use of blood glucose-lowering agents. Hypertension was defined either as the current use of antihypertensive medication or having a measured BP ≥140/90 mmHg at the initial examination. Trained nurses obtained sitting BP levels using a standard mercury sphygmomanometer. The first and fifth Korotkoff sounds were utilized in order to estimate the systolic and diastolic BP.
Blood samples were collected after more than 12 hours of fasting and were drawn from an antecubital vein. Serum levels of aspartate aminotransferase (AST), ALT, and γ-glutamyltransferase (GGT) were measured using the Bayer Reagent Packs (Bayer HealthCare, Tarrytown, NY, USA) on an automated chemistry analyzer (Advia 1650 auto analyzer; Bayer Diagnostics, Leverkusen, Germany). Serum UA was measured using the Fossati enzymatic reaction using uricase with a Trinder-like endpoint (Advia 1650 auto analyzer). High-sensitivity C-reactive protein (hsCRP) was analyzed by performing particle-enhanced immunonephelometry using the BN System (Dade Behring, Marburg, Germany). Insulin levels were measured with immunoradiometric assays (Biosource, Nivelles, Belgium). Insulin resistance was calculated using the HOMA-IR as described by Matthews et al. [9]: fasting serum insulin (μU/mL) × fasting serum glucose (mmol/L) / 22.5.
Serum creatinine (SCr) was measured using the alkaline picrate (Jaffe) method. Renal function was estimated using the glomerular filtration rate (eGFR), which was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation: eGFR = 141 × min (SCr/K, 1)a × max (SCr/K, 1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black], where SCr is serum creatinine, K is 0.7 for females and 0.9 for males, a is -0.329 for females and -0.411 for males, min indicates the minimum of SCr/K or 1 and max indicates the maximum of SCr/K or 1 [10].
Fasting serum glucose was measured using the hexokinase method. Total cholesterol and triglyceride were measured using enzymatic colorimetric tests, low-density lipoprotein (LDL) cholesterol was measured using the homogeneous enzymatic colorimetric test, and high-density lipoprotein (HDL) cholesterol was measured using the selective inhibition method (Bayer Diagnostics).
Body mass index was calculated as the weight (kg) divided by the height squared (m2). A single examiner measured waist circumference (WC) in the standing position and at the level of umbilicus. Height and weight were measured after an overnight fast with the shoeless participants wearing a lightweight hospital gown.
The Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention was used to define MetS in participants with three or more of the following variables [11]. Elevated BP was defined as a systolic or diastolic BP of 130/85 mmHg or higher, elevated fasting serum glucose level was defined as 100 mg/dL or greater, high serum triglyceride level was defined as 150 mg/dL or higher, low HDL-cholesterol level was defined as less than 40 mg/dL (men only), and elevated WC was defined as more than 90 cm (men only) [11].
Statistical Analysis
Data were expressed as means (standard error) or medians (minimum, maximum) for continuous variables and percentages (standard error) for categorical variables. One-way ANOVA and χ2-tests were used to analyze the statistical differences among the characteristics of the study participants at the time of enrollment in relation to the quartile groups of serum UA levels. Serum UA was categorized into the following quartiles: <5.2, 5.3-6.0, 6.1-6.7, and ≥6.8 mg/dL. We stratified our study population by 5-year age groups (from 20-24 to ≥80) and quartile groups of serum UA levels and created standardization weights equal to the size of each stratum. Then, we estimated age-standardized means and proportions for the quartile serum UA level groups using the observed distribution of the stratum as the standard. The p for trend was tested using multiple linear regression models for continuous variables and multiple logistic regression models for categorical variables after adjusting for the 5-year age groups. The distributions of the continuous variables were evaluated, and log transformations were used in the analysis as required.
For incident MetS cases, the time of MetS occurrence was assumed to be the midpoint between the visit at which MetS was first diagnosed and the baseline visit (2005). The person-years were calculated as the sum of follow-up times from baseline until the assumed time of MetS development or until the final examination of each individual, whichever came first. We used Cox proportional hazards models to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for incident MetS comparing the highest three quartiles of baseline serum UA to the lowest quartile (reference). The data were adjusted for the multiple covariates. In the multivariate models, we included variables that might confound the relationship between serum UA and MetS, which included age, total cholesterol, log log(hsCRP), HOMA-IR, eGFR, the number of MetS components at baseline, smoking status, alcohol intake, regular exercise, hypertension, and DM. For the linear trends of risk, the number of quartiles was used as a continuous variable and tested in each model. Cox proportional hazards models were used to check the validity of the proportional hazards assumption. Two approaches were used to assess the validity of the proportional hazards assumption. First, the assumption was assessed by the log-minus-log survival function until it was found to graphically hold. Second, to confirm the validity of the proportional hazards assumption, time-dependent covariate analysis was used. The time-dependent covariate was not statistically significant, suggesting that the proportional hazards assumption was not violated (p=0.145). A p-value <0.05 was considered statistically significant. Statistical analyses were performed using PASW Statistics version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
During 52 466.1 person-years of follow-up, 2428 (16.3%) incident cases of MetS developed between 2006 and 2010. Compared with those who were included in final study population (n=14 906), those who were excluded (n=6507) were 1.4 years older (46.0 vs. 44.6) and had less favorable metabolic profiles at baseline (Supplemental Table 1). In contrast to participants without incident MetS, those with incident MetS tended to be slightly older (44.5 vs. 44.6) and had less favorable metabolic profiles at baseline. As expected, all clinical variables were statistically significant between those who did and did not develop MetS (all p<0.001) (Supplemental Table 2).
The baseline characteristics of the study participants in relation to the quartile groups of serum UA are presented in Table 1. At baseline, the mean (standard error) age and serum UA levels (minimum, maximum) of study participants were 44.6 (0.01) years and 6.0 (0.3, 12.7) mg/dL, respectively. There were clear dose-response relationships between all of the listed variables and quartile groups of serum UA levels except for hsCRP, current smoking status, alcohol intake, and regular exercise. Body mass index, WC, total cholesterol, triglycerides, LDL-cholesterol, HOMA-IR, insulin, SCr, AST, ALT, GGT, hypertension, and the development of MetS were significantly and positively associated with the quartile groups of serum UA levels. However, average person-years, age, HDL-cholesterol, fasting serum glucose, eGFR, and DM were significantly and negatively associated with quartile groups of serum UA levels in our study participants.

Baseline characteristics of participants according to the quartile groups of serum uric acid level (n=14 906)
Table 2 shows the HRs and 95% CI for MetS according to the quartile groups of serum UA levels. In the unadjusted model, the HRs (95% CI) for MetS comparing the second, the third, and the fourth quartile to the first quartile of serum UA levels were 1.13 (1.00-1.28), 1.45 (1.28-1.65), and 2.01 (1.79-2.25), respectively (p for trend <0.001). These associations were apparent even after further adjustments for covariates in models 1 and 2, even though the incidence of the recently updated MetS in the second quartile of serum UA was not significant. In the fully adjusted Cox proportional hazards models (adjusted for age, total cholesterol, log(hsCRP), HOMA-IR, eGFR, the number of MetS components at baseline, smoking status, alcohol intake, regular exercise, hypertension and DM), the adjusted HRs (95% CI) for MetS across the baseline quartile groups of serum UA levels were 1.09 (0.92-1.29), 1.22 (1.04-1.44) and 1.48 (1.26-1.73), respectively (p for trend <0.001).
DISCUSSION
In this prospective study of initially MetS-free, healthy middle-aged Korean men, high serum UA level was significantly and independently associated with a high risk of MetS. During 52 466.1 person-years of follow-up, 2428 incident cases of MetS developed between 2006 and 2010, and the incidence density was 46.3 cases per 1000 person-years. After adjusting for multiple covariates, the HRs (95% CI) for incident MetS comparing the second, the third, and the fourth quartile to the first quartile of serum UA levels were 1.09 (0.92-1.29), 1.22 (1.04-1.44), and 1.48 (1.26-1.73), respectively (p for trend <0.001). Previous prospective studies showed that increased serum UA levels at baseline were associated with a high risk of MetS [6,12-15], and the findings of our study are in accordance with theirs. Moreover, another 3-year follow-up health screening study in Japan demonstrated very similar results to ours; their HRs (95% CI) of MetS was significant only in the fourth quartile of serum UA levels (9.9-10.6 mg/dL) when compared to the first quartile (1.1-5.2 mg/dL) and the HR (95% CI) was 2.206 (1.344-3.620) (p=0.002) after adjusting for relevant multiple covariates [16].
UA is the end-product of purine metabolism in humans that is generated during the metabolism of nucleotides and adenosine triphosphate by the action of xanthine oxidase [17]. Parallel to the findings of previous studies that claimed hyperuricemia might be a new component of MetS, the adjusted HRs (95% CI) for participants with only one component and two components of MetS compared to those with none at baseline were 3.43 (2.79-4.02) and 8.05 (6.57-9.87), respectively. These associations were apparent in the clinically relevant subgroup analysis of our study participants, with BP <130/85 mmHg, fasting serum glucose <100 mg/dL, triglyceride <150 mg/dL, HDL-cholesterol >40 mg/dL and waist circumference <90 cm (Figure 1). A recent review describes the possible mechanism that UA influences the development of CVD by inducing oxidative stress, endothelial dysfunction, inflammation, and vasoconstriction; they also suggest that UA may have a causal role in the development of MetS [18]. UA is a potent antioxidant in extracellular fluid but it also exerts prooxidative effects in the intracellular environment, and it has been demonstrated that serum UA is a circulating marker of oxidant damage in some metabolic disturbances [19]. Increased serum UA levels have been previously reported to be closely associated with hypertension [20-23], DM [23,24], dyslipidemia [25], and abdominal obesity [20], but the HRs of each component of MetS were comparable in the present study.

Adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for the incidence of metabolic syndrome by the serum uric acid level quartiles according to clinically relevant subgroups. HDL, high-density lipoprotein; p trend, p for trend. 1Adjusted for total cholesterol, log(high-sensitivity C-reactive protein), homeostasis model assessment of insulin resistance, estimated using the glomerular filtration rate, number of baseline metabolic syndrome component, current smoking status, regular exercise, alcohol intake, hypertension, and diabetes mellitus.
Even though a positive linear association was observed in our analysis, no significant risk for incident MetS was found in the second quartile (5.3-6.0 mg/dL) of serum UA level. This finding might indicate that the cutoff value of elevated serum UA level that initiates metabolic abnormalities might be within the second quartile; however, the current definition of hyperuricemia for men is 7.0 mg/dL. The optimal cutoff value of serum UA for men as a component of MetS was previously reported as 6.3 mg/dL from a recent 3-year follow-up study of 2957 MetS-free men in China [26], but this value had a low discriminatory power for MetS (area under the curve, 0.601). Thus, further studies are warranted to establish a scientifically proven strategy for use in the clinical setting.
The major strength of our study was that we used a large sample size and excluded for the presence of MetS, CVD, malignancy, and medication use for lipid-lowering agents at baseline. However, when interpreting our results, some limitations should be considered. First, bias from follow-up loss may have affected our results. Participants not included in our analysis (n=6507) tended to be older and had less favorable metabolic profiles at baseline than those in the analytic cohort did. Loss to follow-up is expected, especially in those who are in poor health. However, loss to follow-up of high-risk people would probably lead to a conservative bias and subsequent underestimation of risk. Second, participants were self-selected, so this study may show volunteer bias. Third, our study population was comprised of healthy, middle-aged males, so the findings are unlikely to be generalizable to Korean adults. Fourth, we were not able to include some important confounders in this study, such as the presence of or use of medication to treat gout and any dietary habits.
Additionally, all clinical variables except regular exercise showed statistically significant differences between the groups for the baseline characteristics according to the number of the MetS components they had at baseline (Supplemental Table 3). We believe that this lack of significance for regular exercise might have been caused by a lack of knowledge about the definition of a regular exercise in the health-related behavior questionnaire that was used at the health promotion center. The American College of Sports Medicine and American Heart Association define regular exercise as moderate-intensity daily physical activity for 30 minutes per session five days a week [27], and the US Department of Health and Human Services guidelines focus on completing 150 minutes a week of moderate-intensity aerobic physical activity [28].
In conclusion, our findings, which were obtained from large cohort of Korean men, indicated that serum UA may be a predictor for the development of MetS in a 5-year follow-up period, and this association was significant after adjustment for baseline covariates.
Notes
The authors have no conflicts of interest with the material presented in this paper.