Articles
- Page Path
- HOME > J Prev Med Public Health > Volume 44(4); 2011 > Article
-
Brief Report
Fifteen Years After the Gozan-Dong Glass Fiber Outbreak, Incheon in 1995 - Soo-Hun Cho1, Joohon Sung2, Jonghoon Kim3, Young-Su Ju4, Minji Han2, Kyu-Won Jung3
-
Journal of Preventive Medicine and Public Health 2011;44(4):185-189.
DOI: https://doi.org/10.3961/jpmph.2011.44.4.185
Published online: July 29, 2010
- 6,869 Views
- 55 Download
1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
2Department of Epidemiology, School of Public Health, Seoul National University, Seoul, Korea.
3Department of Cancer Registry, National Cancer Center of Korea, Goyang, Korea.
4Department of Occupational & Environmental Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- Corresponding author: Joohon Sung, MD, PhD. 599 Kwanak-ro Kwanak-gu, Seoul, 151-742, Korea. Tel : +82-2-880-2712 , Fax: +82-2-880-2738, jsung@snu.ac.kr
Copyright © 2011 The Korean Society for Preventive Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Objectives
- In 1995, an outbreak survey in Gozan-dong concluded that an association between fiberglass exposure in drinking water and cancer outbreak cannot be established. This study follows the subjects from a study in 1995 using a data linkage method to examine whether an association existed. The authors will address the potential benefits and methodological issues following outbreak surveys using data linkage, particularly when informed consent is absent.
-
Methods
- This is a follow-up study of 697 (30 exposed) individuals out of the original 888 (31 exposed) participants (78.5%) from 1995 to 2007 assessing the cancer outcomes and deaths of these individuals. The National Cancer Registry (KNCR) and death certificate data were linked using the ID numbers of the participants. The standardized incidence ratio (SIR) and standardized mortality ratio (SMR) from cancers were calculated by the KNCR.
-
Results
- The SIR values for all cancer or gastrointestinal cancer (GI) occurrences were the lowest in the exposed group (SIR, 0.73; 95% CI, 0.10 to 5.21; 0.00 for GI), while the two control groups (control 1: external, control 2: internal) showed slight increases in their SIR values (SIR, 1.18 and 1.27 for all cancers; 1.62 and 1.46 for GI). All lacked statistical significance. All-cause mortality levels for the three groups showed the same pattern (SMR 0.37, 1.29, and 1.11).
-
Conclusions
- This study did not refute a finding of non-association with a 13-year follow-up. Considering that many outbreak surveys are associated with a small sample size and a cross-sectional design, follow-up studies that utilize data linkage should become standard procedure.
- An outbreak survey at the Gozan-dong, Inchon area was initiated by a suit of health hazard filed by the local residents in 1994. Glass fibers, dispersed from a glass fiber insulation material factory were the alleged cause of health hazards including stomach cancer. An association between stomach cancer and glass fiber intake through contaminated water was reported [1], which had escalated the case into a public health issue. A large scale survey was launched by the support of the Incheon city government ("the survey") [2,3]. Unlike asbestos, health effects of glass fiber had been largely unknown, and the survey team developed methods of exposure assessment with international collaboration [4,5]. In the survey, main exposure of interest was the extent of glass fiber intake through contaminated water [2]. Through analyses on the drinking water sources, 31 individuals were classified as exposed. The survey team in 1995 consisted of multidisciplinary expertise [6-9], which became a role model for similar investigation [10,11]. The survey, however, had too few exposed subjects, so that the negative results could be either interpreted as small effect size or as lack of power. The issue of underpower study was not limited to this survey, but a general problem of local outbreak surveys because of the limited sample size and the cross-sectional designs [6,10,12]. It is often infeasible to confirm or refute alleged associations by the initial survey alone [13,14].
- Previous studies, including the survey in 1995, were conducted without getting written informed consent. However, if personal information is available for research purposes, current policy of public institutes allows researchers to generate group statistics after deleting individual level information. In Korea, numerous epidemiologic studies have established associations between risk factors and diseases by using open data sources or data linkage methods. However, in the field of environmental epidemiology, these efforts have been very limited. We are determined to follow the 1995 participants by rehabilitating the personal information of baseline survey. There were some methodological issues as well as ethicolegal considerations when follow-up studies were performed using data without individual consent. The authors will present the findings from the follow-up study, as well as involved methodological issues so that our experience can be used in similar studies.
INTRODUCTION
- I. Participants
- The same criteria for classifying exposure status were used as the initial survey, assuming that additional exposure was absent, because the glass fiber factory was shut down in 1995 [2,3]. Among the 31 exposed and 858 control groups, those with valid personal ID number were involved in this study. The definition of exposure and control group are as follows: those using contaminated water by glass fiber were defined as exposed (n=31); external control group (=control 1) included those who lived nearby, but were not a party to the glass fiber suit (n=642); internal control group (control 2) were the same residents active in the case, but their drinking water were clean from glass fiber contamination (n=215). We assumed that comparisons between exposure and external control group will reflect both the effects of glass fiber and possible interests for compensation; the comparisons between exposure and internal control group will capture the health effects of glass fiber only.
- II. Methods of Follow-Up
- After acquiring the approval from the institutional review board, we sent the personal ID of all available data to the National Cancer Registry of National Cancer Center Korea, and the Korean Statistical Office, where follow-up for cancers and mortality was performed. The follow-up period started from 1995 until the end of 2007 (13 years). The cancer and mortality occurrences during the period were provided as indirectly standardized rates by the exposure status, after deleting personal information. We selected disease codes for cancers as C00-C97 and gastrointestinal tract cancers C15-C20 by International Classification of Disease and Related Health Problems 10th revision (ICD-10).
- III. Standardized Incidence Ratio and Standardized Incidence Mortality Ratio Calculation
- During the 13 year follow-up period, we reconstructed cumulative observation time as person*year, following the increase of age (5 year window). For example, if there was a 63-year old man at the survey, he contributed two years (=two person*year) to the 60-64 group between 1995 and 1996; his contribution to this 60-64 group is confined to two years and he had contributed 5 years to 65-69 group between the 1997 and 2001, and to the next age group on. By this manner, the cumulative person*time for calculating standardized rates was calculated considering age and sex. We have conducted indirect standardization of the incidence and the mortality rates based on the rates of 2001 mortality in Koreans, and 2001 end-of-year census population structure.
- Standardized incidence ratio (SIR) and standardized mortality ratio (SMR) were calculated by dividing the actual observed cases and deaths by expected number of deaths estimated from the population rates. 95% confidence intervals (CIs) of the standardized rates were calculated with the method by Breslow and Day (Formula 1) [15]
- Formula 1: 95% CIs of standardization rate
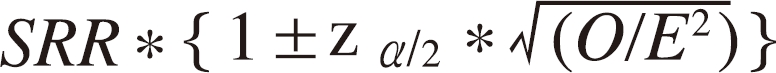
- Considering the difference in the follow-up loss rate between the exposed and control group, we estimated corrected SIR for controls, assuming that the no more cancer cases were occurred with complete follow-up. The corrected SIR, thus, is the least possible SIR level, and if the exposed group's SIR is lower than the corrected SIR, we can exclude the possibility that SIR is "higher" among the exposed.
METHODS
- Among the original 888 participants, personal IDs of the 697 individuals (78.5%) were valid. The number of followed subjects according to their exposure status was presented in Table 1. While all subjects except one person were included in the exposure group, the external and internal control groups showed follow-up rates of 76.2% and 82.7%. When we compared the sex ratio, smoking and drinking prevalence, those followed in the internal control group (control 2) had more male, more smokers and heavy drinkers than those lost in the same group, but those in exposed and external control group did not show significant differences between the followed and lost.
- SIRs of all types and digestive system cancer were estimated as follows; 0.73 (95% CI, 0.10 to 5.21) and 0.00 (no cases) for the exposed group; 1.18 (95% CI, 0.82 to 1.71) and 1.62 (95% CI, 0.96 to 2.74) for the external control group; 1.27 (95% CI, 0.64 to 2.55) and 1.46 (95% CI, 0.47 to 4.52) for the internal control group (Table 2). The corrected SIRs assuming complete follow-up were 0.92 in the external control group.
- The SMRs from all cause death were 0.37 (95% CI, 0.05 to 2.67), 1.29 (95% CI, 0.78 to 2.15) and 1.11 (95% CI, 0.84 to 1.45) for the exposed, external and internal control groups. SMR in the exposed was lower than two control groups without statistical significance. (Table 3)
RESULTS
- The findings from 13 year follow-up did not refute the non-association. When we compare the results of this follow-up study and those in 1995, the original trend of higher SIR and SMR in the exposed were reversed, although all the results were not statistically significant.
- There are two explanations for the lack of significance; insufficient power and true non-associations. If we assume 50 year follow-up for 30 and 600 individuals in the exposed and controls, and 30% life time risk of all cancers in control group, the effect size should exceed 2.0 to be significant (with 80% power and 5% alpha error level). Another explanation is the lack of association or very small effect size. If the representative estimators of SIRs and SMRs are higher in the exposed group, we cannot conclude whether the findings are derived from insufficient power or smaller than detectable effect size (or lacking associations). The lower SIRs and SMR in the exposed group, however, disapprove of "increased risk" in the exposure group although it cannot prove that "smaller risk".
- For the reversed cancer SIRs between the exposed and controls compared with those ratios in 1995, several explanations are possible. First, the 1995 survey, as a cross-sectional study, could have been influenced by information errors. Over- or under-report between two groups were possible because the SIR of cancers solely depended on the questionnaire in 1995. Second, chance event may explain some part of the findings. Another possibility is the "harvesting effect". If an excess of mortality actually existed in a small group of population, the excess deaths will lead to reduced mortality in near future. However, it is unlikely that both of the lower SIR and SMR can be explained by the harvesting effect alone, considering the relatively long follow-up period. The harvesting effects are well documented in time-series analyses of air pollution health studies. The methodologies to adjust and detect the harvesting effects in small group follow-up study remain as future tasks.
- Some Methodological issues are worth addressing. First, methods of handling the difference in the follow-up rate by the exposure status should be considered. In this study we had lower follow-up rate in the controls and we estimated corrected SIRs in order to calculate the minimum SIR level. The method of calculating corrected SIRs can be different depending on the situation. If the exposed have higher SIRs, the corrected SIR in the control should assume "maximum" SIR by applying the highest level of incidence rate (=upper CI of the SIR) to the lost, and that in the exposed be "minimal". In this study by showing that the SIRs in the exposed were lower than the minimum corrected SIR of the control, we could conclude that the SIRs in the exposed is "not higher" than those in control group. This sensitivity analysis can be applied to draw logical inferences out of the incomplete data.
- Second, an ethicolegal issue for the follow-up study; is it possible to follow the previously acquired study participants who do not have submitted explicit informed consent for data linkage? The concept of written informed consent was not settled in 1995. The authors did provide written information to the participants and the participation itself was regarded as consent at that time. It is, however, not sufficient under current ethical standard. Current data management policy of The National Cancer Registry (KNCR) reconciles the insufficient consent and research needs by providing aggregate data after deleting any personal information when the purpose of research is approved. The current policy of protecting privacy, however, will limit some types of analysis, particularly the cause-specific mortality analysis. In addition, information on the rare type of cancer occurrences will not be provided by the same token. It will be an issue of research ethics whether the cause-specific mortality or rare cancer can be provided as a statistical data or not.
- Vital statistics data and cancer registry data in Korea are well-documented for their validity and reliability [16-18]. Until the late 1990s, about 20% of death records in the vital statistics have lacked the diagnosis of the medical doctors [16]. Deaths from cancer, however, have higher rate of medical certificate [17], and it is unlikely that the SMRs from cancer will be affected by the quality of vital statistics before late 1990s. The KNCR data since the mid 1990s have met the international standard in terms of completeness and validity. [18]
- In the 1995 survey, the authors indicated the possible increase in benign soft tissue tumors in the exposed. This finding, however, could not be examined by the current data linkage method. When the health effects of interest are subclinical pathologic changes, systematic biobank will be helpful. Regardless of the initial decision, further knowledge will allow new approaches to examine the associations using collected biospecimens. In the survey of 1995, although efforts to collect some biospecimens had been attempted, the limitations of quality and quantity did not allow new analyses. The authors suggest the long-term follow-up become a standard practice of any outbreak epidemics. Additionally, we suggest that the construction of systematic biobank, as well as getting informed consents for data linkage, be included in the standard practice of outbreak survey to improve the current ability to detect or refute associations.
DISCUSSION
-
The authors have no conflicts of interest with the material presented in this paper.
-
This article is available at http://jpmph.org/.
Notes
- 1. Lim HS, Cheong HK, Kim JY, Cheong HK, Kim JR, Hong YC, et al. An epidemiologic study on the health hazards of inhabitants chronically exposed to glass fiber. Korean J Epidemiol 1995;17(1):76-93. (Korean)
- 2. Cho SH, Hong JW, Kwon HJ, Kim KR, Song DB, Yoo KY, et al. Final report of epidemiologic survey on health effects of fiber glass in gozan-dong, Incheon. 1995. Incheon: National Institute of Environment; (Korean)
- 3. Cho SH, Ju YS, Kim KR, Lee KK, Hong KS, Eum HC, et al. Health assessment for glass fibre landfill at gozan-dong, Inchon. Korean J Prev Med 1997;30(1):77-101. (Korean)
- 4. World Health Organization. IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Man-made mineral fibres and radon. International Agency for Research on Cancer
- 5. Lee SW, Kim KS, Choi JK, Kim YH, Kang SK, Choi KS, et al. Medical surveillance of glass fiber workers in Korea. Korean J Prev Med 1996;29(2):187-198. (Korean)
- 6. Ahn YO, Park BJ, Bae JM, Lee DH, Kim DJ, Kim JH, et al. An epidemiologic survey on cancer epidemic at pukcheju-gun. Korean J Epidemiol 1993;15(2):185-195. (Korean)
- 7. Park BJ, Bae JM, Ahn YO, Yoo KY. Survey methods on cancer epidemic. Korean J Prev Med 1994;27(3):411-424. (Korean)
- 8. Cho SH. Hazardous health effects of environmental pollution. Korean J Prev Med 1995;28(2):245-258. (Korean)
- 9. Cho SH, Kim SM, Cho SI. Environmental pollution related health problems reported in newspapers. Korean J Prev Med 1993;26(1):126-146. (Korean)
- 10. Chung JH, Kang PS, Kim CY, Lee KS, Hwang TY, Kim GT, et al. Blood Pb, urine Cd and health assessment of residents in the vicinity of abandoned mines in Gyeongsangbuk-do. Korean J Occup Environ Med 2005;17(3):225-237. (Korean)
- 11. Kim YM, Cheong HK, Kim JH, Kim JH, Ko K, Ha M. Scientific basis of environmental health contingency planning for a coastal oil spill. J Prev Med Public Health 2009;42(2):73-81. (Korean). 19349735ArticlePubMedPDF
- 12. Cho SH, Choi SW, Kim SM, Ju YS, Kim JY. Health effects from odor pollution in sihwa industrial complex. Korean J Prev Med 1999;32(4):473-481. (Korean)
- 13. McBride ML, Gallagher RP, Thériault G, Armstrong BG, Tamaro S, Spinelli JJ, et al. Power-frequency electric and magnetic fields and risk of childhood leukemia in Canada. Am J Epidemiol 1999;149(9):831-842. 10221320ArticlePubMed
- 14. Draper G, Vincent T, Kroll ME, Swanson J. Childhood cancer in relation to distance from high voltage power lines in England and Wales: a case-control study. BMJ 2005;330(7503):1290. 15933351ArticlePubMedPMC
- 15. Breslow NE, Day NE. In: Sen PK, Greenberg BG, editors. The standardized mortality ratio. Biostatistics: statistics in biomedical, public health, and environmental sciences: the Bernard G. Greenberg vol. 1985. Amsterdam: North-Holland; p. 55-74
- 16. Korea National Statistical Office. Annual report on the cause of death statistics 2000. 2001. Daejeon: Korea National Statistical Office; (Korean)
- 17. Ahn YO. Cancer registration in Korea: the present and furtherance. J Prev Med Public Health 2007;40(4):265-272. (Korean). 17693728ArticlePubMedPDF
- 18. Ministry for Health, Welfare and Family Affairs. Annual report on the national cancer registry of the Korea 2007. 2009. Seoul: Ministry for Health, Welfare and Family Affairs; (Korean)
REFERENCES
| Classification | No. of subjects completed surveys in 1995 | No. of subjects followed (follow-up rate %) | No. subjects with insufficient ID information | |||
|---|---|---|---|---|---|---|
| Exposed | A: 35.0 | A: 35.4 | A: 33.0 | |||
| 31 | M: 38.7 | 30 (96.7) | M: 40.0 | 1 | M: 0.0 | |
| S: 29.0 | S: 26.6 | S: 0.0 | ||||
| D: 0.0 | D: 0.0 | D: 0.0 | ||||
| Control 1 | A: 32.81 | A: 33.71 | A: 26.21 | |||
| 642 | M: 46.8 | 489 (76.2) | M: 46.6 | 153 | M: 48.4 | |
| S: 34.3 | S: 35.1 | S: 31.2 | ||||
| D: 24.4 | D: 26.2 | D: 19.0 | ||||
| Control 2 | A: 39.11 | A: 39.21 | A: 38.7 | |||
| 215 | M: 46.0 | 178 (82.7) | M: 49.7 | 37 | M: 21.71 | |
| S: 34.4 | S: 36.7 | S: 15.71 | ||||
| D: 22.7 | D: 24.0 | D: 11.11 | ||||
| Total | A: 37.4 | A: 37.6 | A: 36.8 | |||
| 888 | M: 46.5 | 697 (78.5) | M: 46.9 | 191 | M: 44.5 | |
| S: 34.2 | S: 35.1 | S: 29.3 | ||||
| D: 24.2 | D: 26.2 | D: 18.6 | ||||
A: mean age (years), M: men %, S: smoker %, D: drinker %.
Bold: significant difference between followed and not-followed subjects in each exposure group (p<0.05, by chi-square test, d.f.=1).
1 significantly different distribution compared with the similar stratum of other exposure status (p<0.05, by chi-square test d.f.=2).
| Group (n) | Observed case | Person-year of observation | Expected No. of cases | SIR (95% CI) | SIR adjusted for f/u rate4 |
|---|---|---|---|---|---|
| Exposed (30) | All cancer2 1 | 374.8 | 1.36 | 0.73 (0.10 - 5.21) | 0.70 |
| GI cancer3 0 | 380.9 | 0.46 | NA | ||
| Control 11 (489) | All cancer 29 | 5932.5 | 24.5 | 1.18 (0.82 - 1.71) | 0.92 |
| GI cancer 14 | 5984.1 | 8.62 | 1.62 (0.96 - 2.74) | ||
| Control 21 (178) | All cancer 8 | 2191.3 | 6.29 | 1.27 (0.64 - 2.55) | 1.07 |
| GI cancer 3 | 2192.9 | 2.06 | 1.46 (0.47 - 4.52) | ||
| All subjects (697) | All cancer 38 | 8498.6 | 32.2 | 1.18 (0.86 - 1.62) | NA |
| GI cancer 17 | 8558.0 | 11.2 | 1.53 (0.95 - 2.45) |
SIR: standardized incidence ratio of cancer occurrence, CI: confidence interval, f/u: follow up, NA: not available.
1 control 1: external control, control 2: internal control,
2 all cancer cases including ICD-10 code C00-C97,
3 all gastrointestinal cancer cases including ICD-10 code C15-C20,
4 adjusted SIR: SIR calculated assuming all the subjects were followed-up and no more cases were found (minimal SIR).
Figure & Data
References
Citations

 KSPM
KSPM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite

